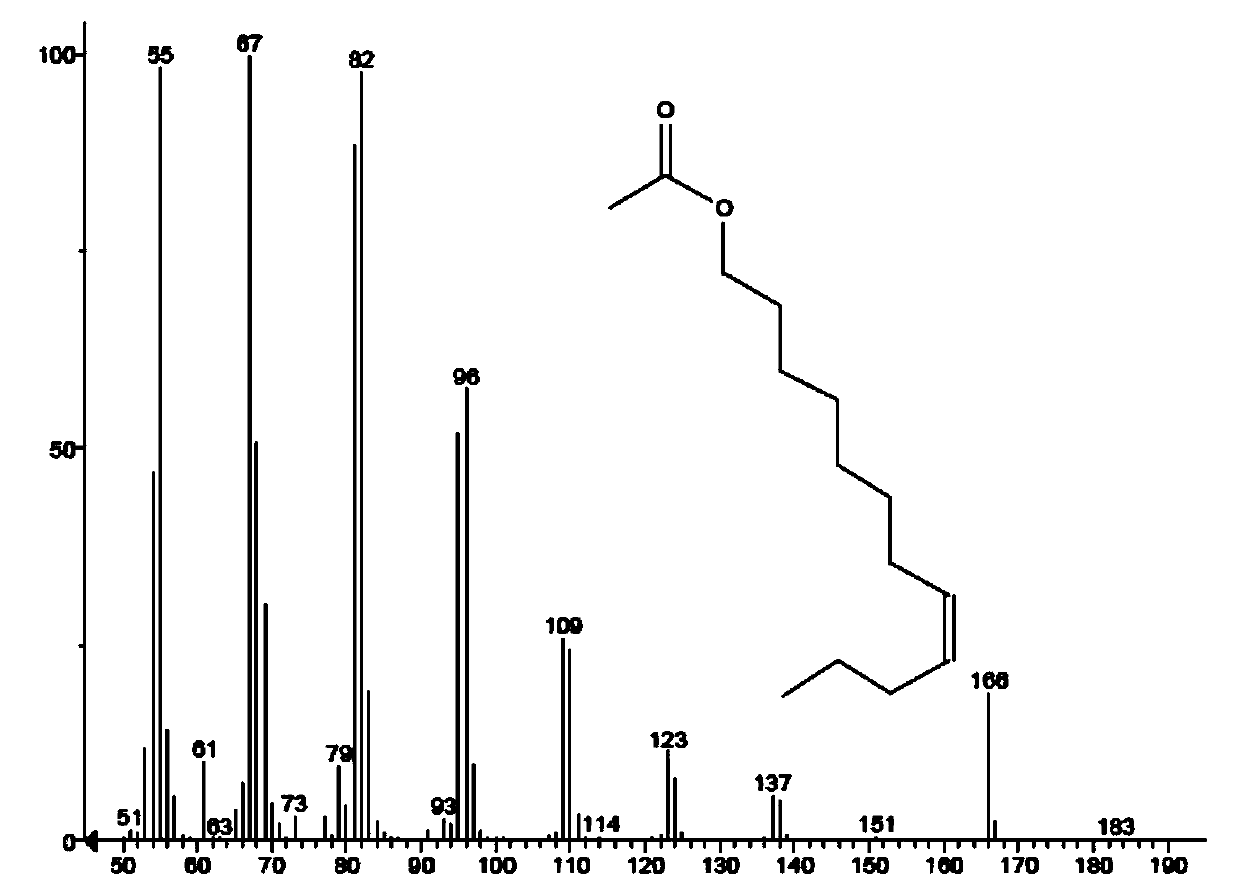
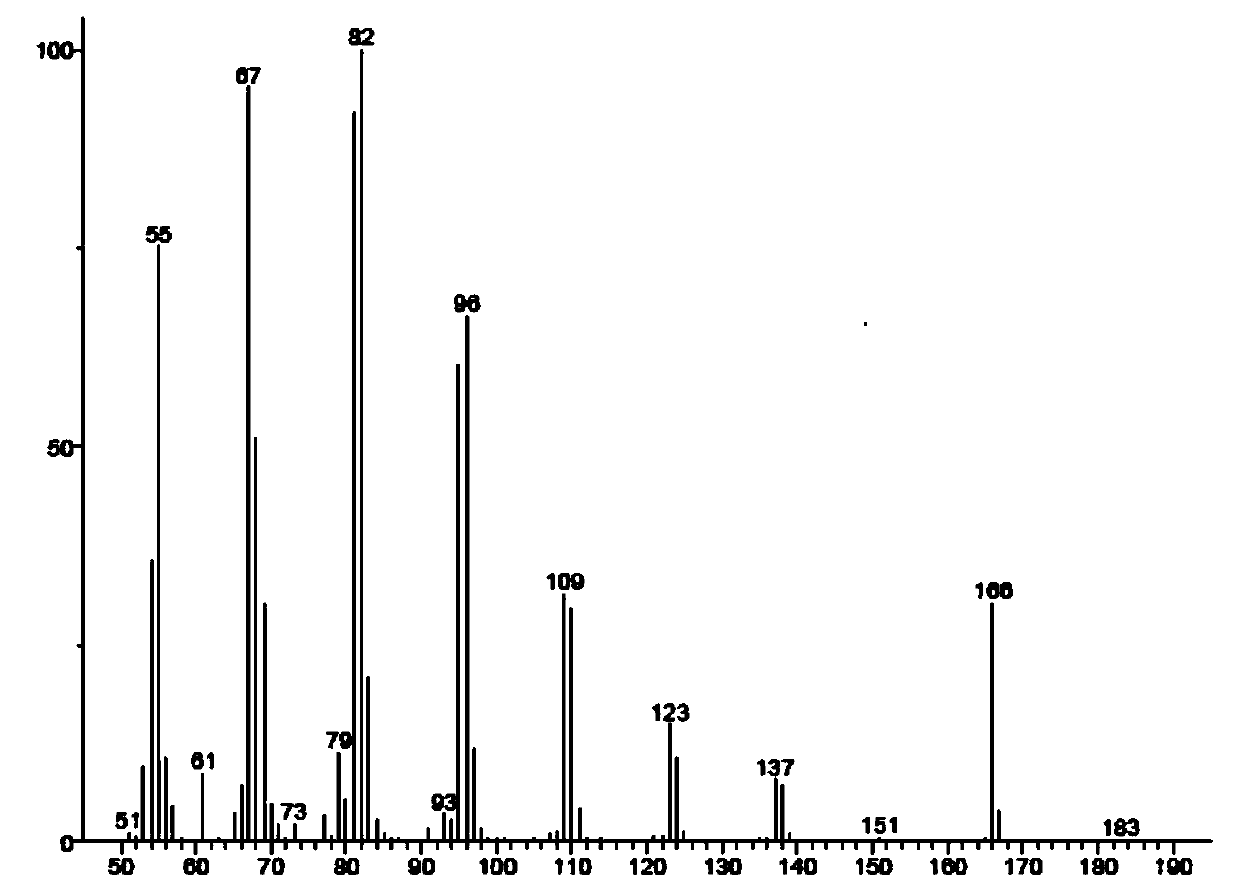
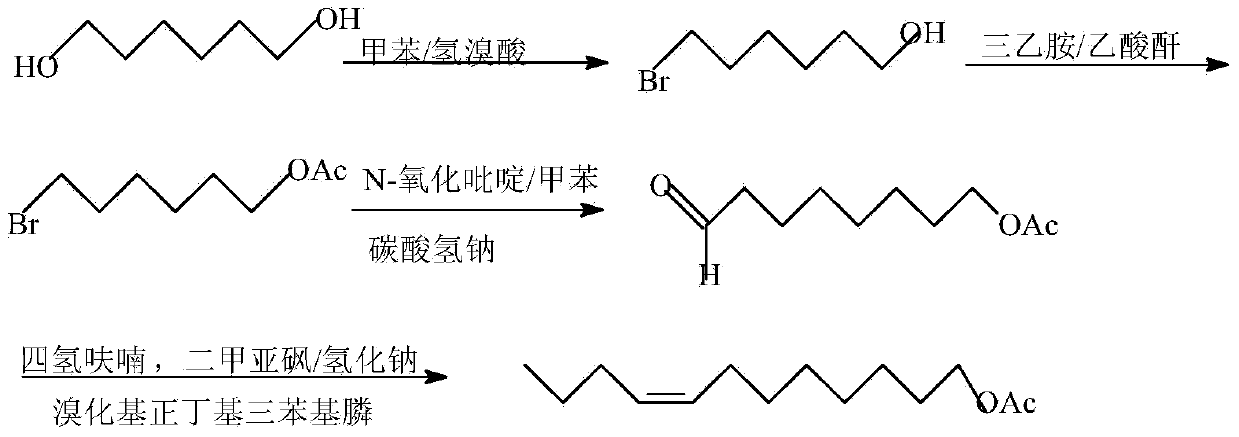
Synthesis method of grapholitha molesta sex pheromone (Z/E)-8-dodecene acetate
The invention relates to a technology for the sex pheromone and synthesis method of the pear worm, which is applied in the field of chemical synthesis of the sex pheromone of Lepidoptera pests, can solve the problems of harsh operating conditions, high cost, easy explosion and the like, and achieves mild conditions and high yield. , the effect of fewer steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] a. 8-Bromo-1-octanol synthesis
[0032] 1,8-octanediol (1.46kg, 10mol) and toluene (25L) were added to the reactor, stirred and heated to 80-90°C, and 47% of pale yellow HBr (1.9Kg, 11mol) was added dropwise in four times. ), every half an hour, the mol ratio of four times of dropping HBr was 10:5:3:2, after the dropping was completed, the samples were incubated for 2 hours for sampling analysis; TLC monitoring, when 1,8-dibromooctane When the content reaches 3%, it is the end point of the reaction, cool to normal temperature, separate layers, wash the organic layer 3 times with 5% NaOH, 10% HCl, water, and saturated sodium bicarbonate solution in turn, until neutral, add Na 2 SO 4 Dry overnight. The solvent was removed with a rotary evaporator to obtain 1.78kg of 8-bromo-1-octanol, the yield of the product was 81%, and the content was >95% by GC inspection.
[0033] b. Synthesis of 8-hydroxyoctyl triphenylphosphine salt
[0034] Add 8-bromo-1-octanol (1.7kg, 8.1mol...
Embodiment 2
[0040] a. 8-Bromo-1-octanol synthesis
[0041] Add 1,8-octanediol (1.02kg, 7mol) and benzene (18L) to the reactor, stir and heat up to 80-90°C, slowly add 47% light yellow HBr (1.33Kg, 7.7mol) dropwise , after the dropwise addition, the temperature was kept for 3 hours for sampling and analysis; TLC monitoring, when the content of 1,8-dibromooctane reached 3%, was the end of the reaction, cooled to room temperature, and layered, respectively, with 5% NaOH, 10% HCl, The organic layer was washed 3 times with water and saturated sodium bicarbonate solution successively until neutral, and Na 2 SO 4 Dry overnight. The solvent was removed with a rotary evaporator to obtain 1.07kg of 8-bromo-1-octanol, the yield of the product was 73.1%, and the content was >95% by GC inspection.
[0042] b. Synthesis of 8-hydroxyoctyl triphenylphosphine salt
[0043] 8-Bromo-1-octanol (1kg, 4.78mol), triphenylphosphine (1.89kg, 7.17mol) and absolute ethanol (2.5L) were added to the reactor and hea...
Embodiment 3
[0049] a. 8-Bromo-1-octanol synthesis
[0050] 1,8-octanediol (0.73kg, 5mol) and toluene (12.5L) were added to the reactor, stirred and heated to 80-90°C, and 47% of light yellow HBr was slowly added dropwise twice at intervals of half an hour. (0.95Kg, 5.5mol), after the dropwise addition was completed, the temperature was kept for 2 hours for sampling and analysis; TLC monitoring, when the 1,8-dibromooctane content reached 3%, was the reaction end point, cooled to room temperature, and layered, using 5 %NaOH, 10% HCl, water, and saturated sodium bicarbonate solution were successively washed 3 times for each organic layer until neutral, and Na 2 SO 4 Dry overnight. The solvent was removed with a rotary evaporator to obtain 0.87kg of 8-bromo-1-octanol, the product yield was 83.5%, and the content was >95% by GC inspection.
[0051] b. Synthesis of 8-hydroxyoctyl triphenylphosphine salt
[0052] 8-bromo-1-octanol (0.8kg, 3.82mol), triphenylphosphine (1.6kg, 6mol) and dehydr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com