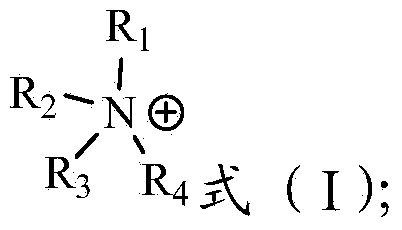Extraction separation method for heavy rare earth elements
A technology of heavy rare earth elements and a separation method, which is applied in the field of extraction and separation of heavy rare earth elements, can solve the problems of high concentration of heavy rare earth elements stripping acid, difficulty in obtaining high-purity heavy rare earth products, and small separation coefficient of rare earth elements, etc. Good interface phenomenon, saving acid and alkali consumption, high selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Synthesis of ionic liquid-based extractant: take 0.6 mole of methyltri-n-hexylammonium bromide [N1666][Br], dissolve it in 1L of methanol, add a strong basic anion exchange resin, exchange bromide ions into hydroxide ions, and obtain hydrogen Methyltri-n-hexylammonium oxide [N1666][OH]. Add 0.6 moles of 2,4,4-trimethylpentylphosphonic acid-mono-2,4,4-trimethylpentyl ester (code P572) dropwise into the generated [N1666][OH] to complete deprotonation to obtain ionic liquid-based extractant [N1666][P572].
[0059] Preparation of organic phase: Mix [N1666][P572] with sulfonated kerosene to form an organic phase. The volume ratio of ionic liquid-based extractant to sulfonated kerosene in the organic phase is 0.1:0.9.
[0060] Preparation of raw material solution: take Tm-Yb-Lu enrichment, add dilute hydrochloric acid, and prepare raw material solution, its composition is ΣREO=0.02 mol / liter, NaCl=0.5 mol / liter, pH=2.2, Tm:Yb:Lu = 10:80:10.
[0061] Single-stage extraction...
Embodiment 2~8
[0071] Preparation of loaded organic phase: Mix [N1666][P572] prepared in Example 1 with sulfonated kerosene to form an organic phase. The volume ratio of ionic liquid-based extractant to sulfonated kerosene in the organic phase is 0.02:0.98. with LuCl 3 Extraction was performed with a loading of 0.53 g / L in the organic phase.
[0072] Preparation of back-extraction solution: prepare a series of hydrochloric acid as back-extraction solution with concentrations of 0.05, 0.1, 0.125, 0.15, 0.2, 0.25 and 0.3 mol / liter.
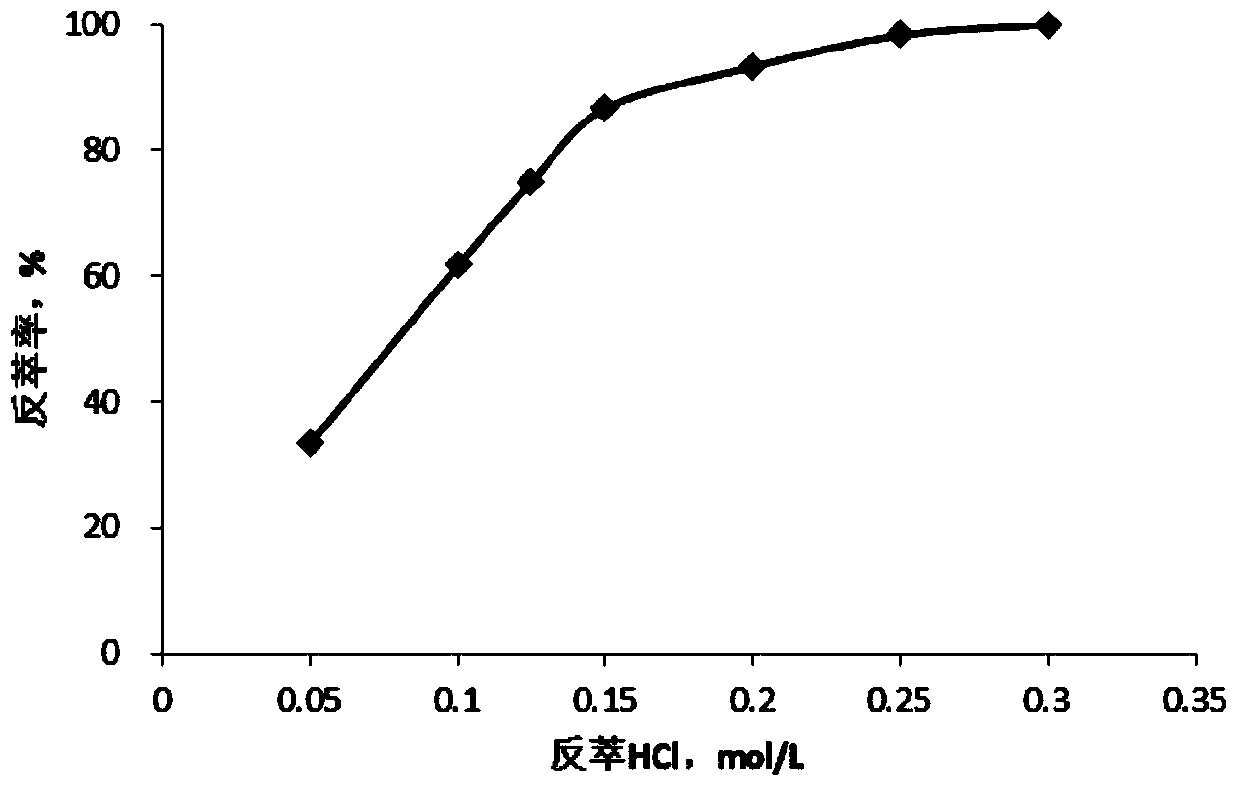
[0073] Single-stage stripping: Mix the loaded organic phase with a volume ratio of 1:1 and the stripping liquid, and strip at room temperature, and the stripping stage is 1 stage. After the extraction was completed, the stripping rate was calculated. The result is as figure 1 as shown, figure 1 It is the stripping result diagram provided by Examples 2-8 of the present invention. Depend on figure 1 It can be seen that the ionic liquid-based extractant is easy...
Embodiment 9-12
[0075] Synthesis of ionic liquid-based extractant: Take 0.6 mole of tetrabutylammonium bromide [N4444][Cl], dissolve it in 0.4 liter of ethanol, add dropwise 305 milliliters of 2 moles of potassium hydroxide-ethanol solution per liter, stir for 0.5 hours and then filter Precipitation of potassium chloride gave tetrabutylammonium hydroxide [N4444][OH]. Mix [N4444][OH] with 2,3,4-trimethylpentylphosphonic acid-mono-2,3,4-trimethylpentyl ester (code P573) to complete deprotonation to obtain ionic liquid Base extractant [N4444] [P573].
[0076] Prepare the organic phase: mix [N4444][P573] with sulfonated kerosene to form the organic phase, and the molar concentration of the ionic liquid-based extractant in the organic phase is 0.03 moles per liter.
[0077] Preparation of raw material solution: take GdCl separately 3 , TbCl 3 , DyCl 3 , HoCl 3 , ErCl 3 0.0045 moles per liter, adjust pH 2.2.
[0078] Single-stage extraction: The organic phase and the raw material solution ar...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com