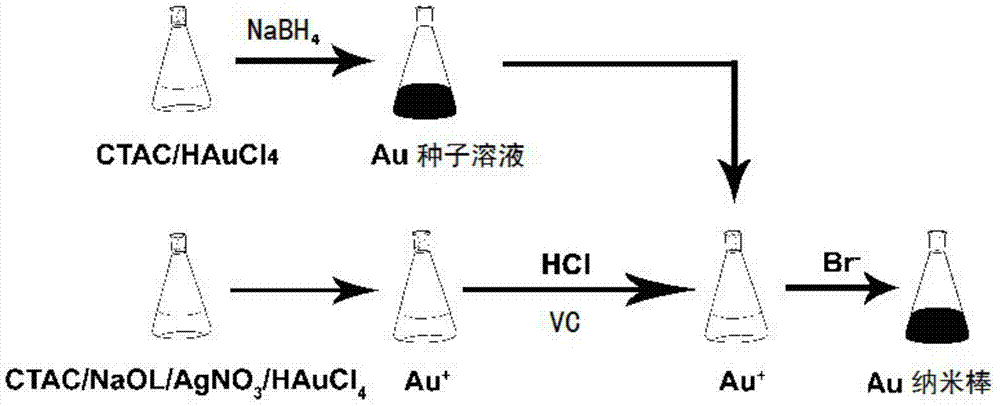
Method for rapidly preparing gold nanorod
A technology for preparing gold nanorods, which is applied in the field of nanomaterials, can solve the problems of long growth cycle, reduce reduction speed, and limit the application value of gold nanorods, and achieve the effects of less spherical particles, high yield, and shortened synthesis time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] In this embodiment, the quaternary ammonium chloride is preferably cetyltrimethylammonium chloride. The soluble silver ion solution is preferably a silver nitrate solution. The acid solution is preferably concentrated hydrochloric acid with a mass fraction of 37%. The bromide ion solution is preferably a potassium bromide solution.
[0039] A. Preparation of growth solution: Weigh 6.145g of cationic surfactant CTAC and 1.234g of sodium oleate and dissolve them in 250mL of ultrapure water, heat and stir at 30°C until completely dissolved. This process lasts for about 2 hours. Vigorous stirring is not suitable during this process, and the degree of stirring is subject to the fact that the solution does not foam; then add 18mL, 4mM silver nitrate solution, and continue stirring for 15 minutes to reach equilibrium; then add 250mL, 1mM HAuCl 4 Solution, at this time, the solution is golden yellow, continue to keep heating and stirring until the yellow color of the solution...
Embodiment 2
[0045] This embodiment provides a method for rapidly preparing gold nanorods. The method of this embodiment is basically the same as the method for preparing gold nanorods described in Embodiment 1, and will not be repeated here.
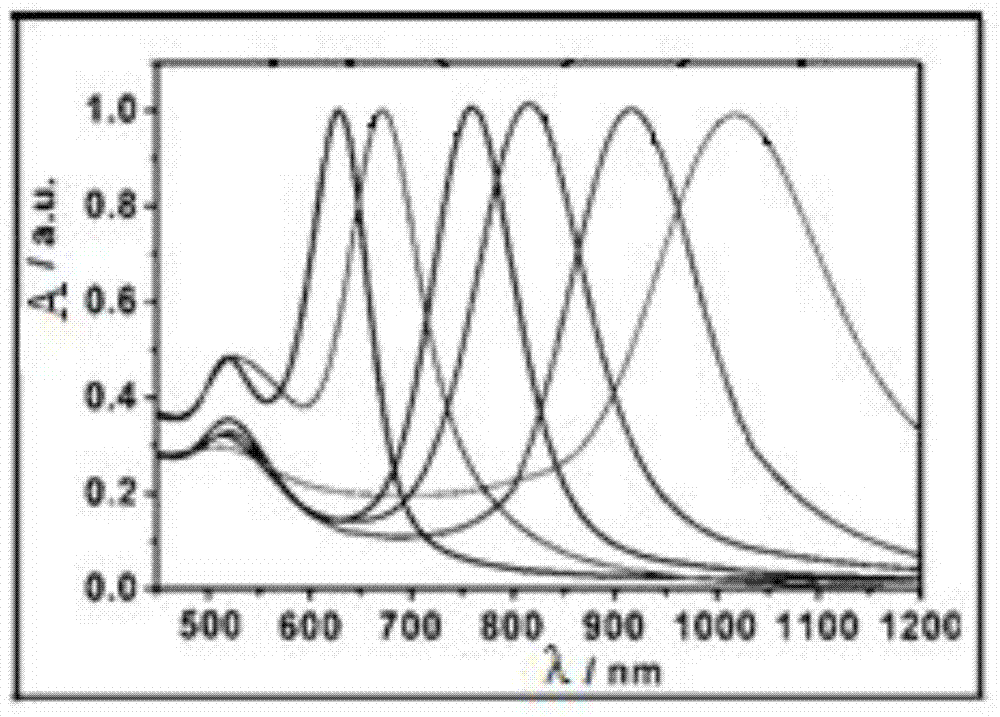
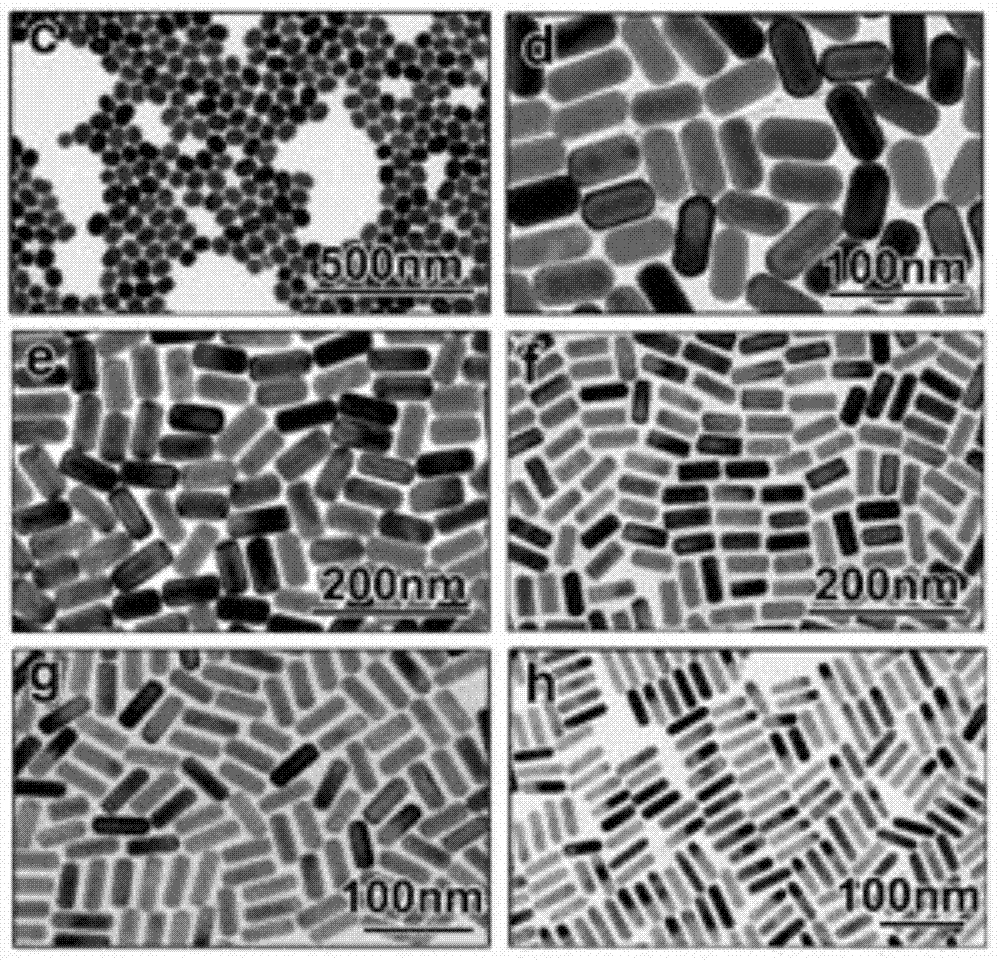
[0046] The difference is that in this embodiment, the concentration of the silver nitrate solution is set as a concentration gradient, and multiple groups of comparative experiments can be carried out through the same method of preparing gold nanorods, and the gold nanorods prepared by using different concentrations of silver nitrate solutions can finally be obtained Gold nanorods with different aspect ratios.
[0047] figure 2 It is a comparison chart of ultraviolet-visible absorption spectra of gold nanorods with different aspect ratios prepared in this example. From figure 2 It can be seen that the transverse plasmon resonance absorption peaks of gold nanorods with different aspect ratios are all located at a wavelength of about 520nm, and th...
Embodiment 3
[0051] This embodiment provides a method for rapidly preparing gold nanorods. The method of this embodiment is basically the same as the method for preparing gold nanorods described in Embodiment 1, and will not be repeated here.
[0052] The difference is that in this embodiment, the CTAC of the same molar concentration in Embodiment 1 is replaced with the chlorine-containing cationic surfactant dodecyltrimethylammonium chloride (DTAC) or tetradecyl trimethylammonium chloride (DTAC) of different chain lengths respectively. Methylammonium chloride (TTAC) or octadecyltrimethylammonium chloride (OTAC), multiple groups of comparative experiments can be carried out by the same preparation method of gold nanorods, and the gold prepared by different quaternary ammonium chloride solutions Nanorods can finally obtain gold nanorods with different shapes and sizes.
[0053] Figure 5 It is a comparison chart of ultraviolet-visible absorption spectra of gold nanorods prepared by DTAC, T...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com