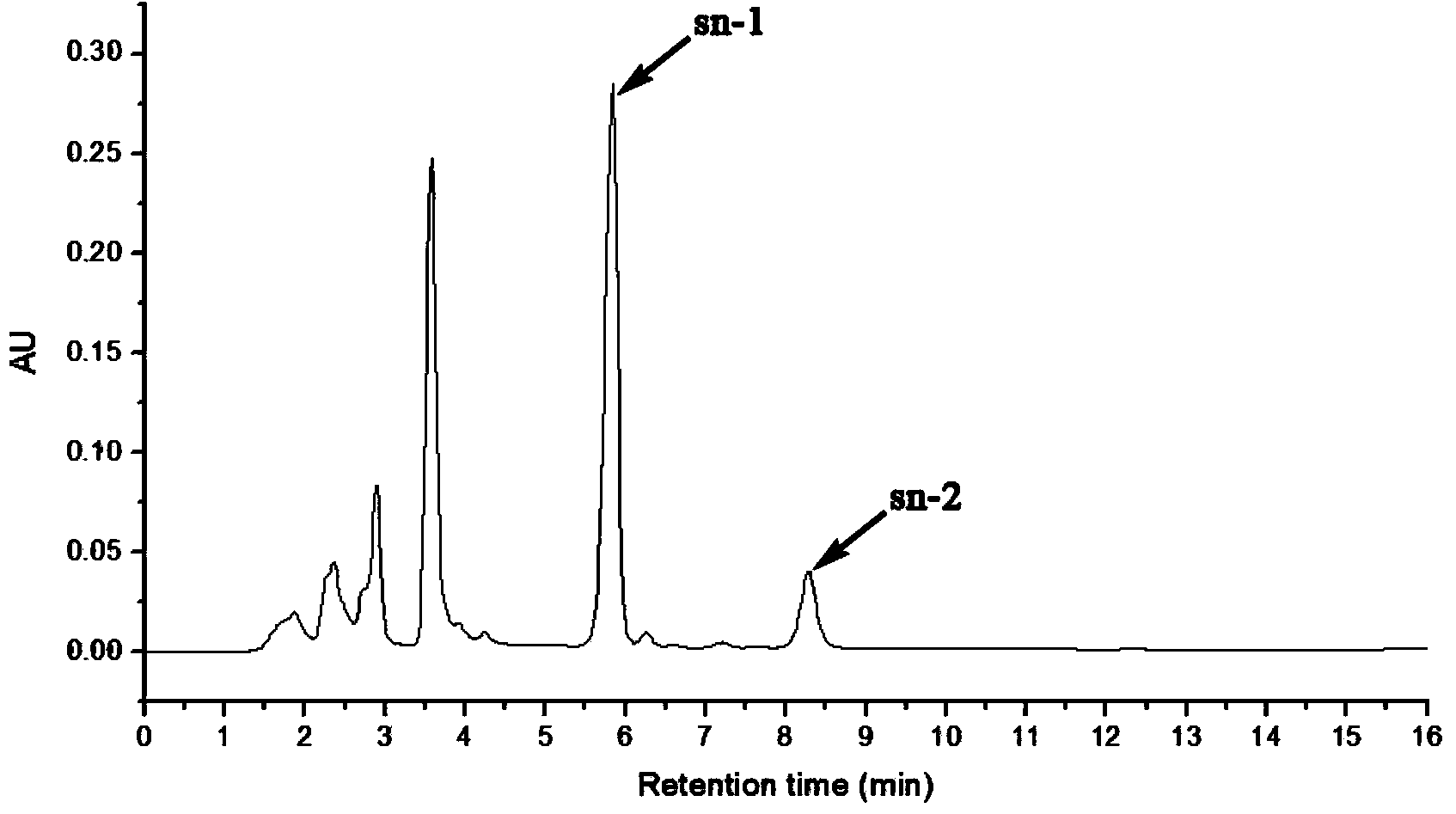
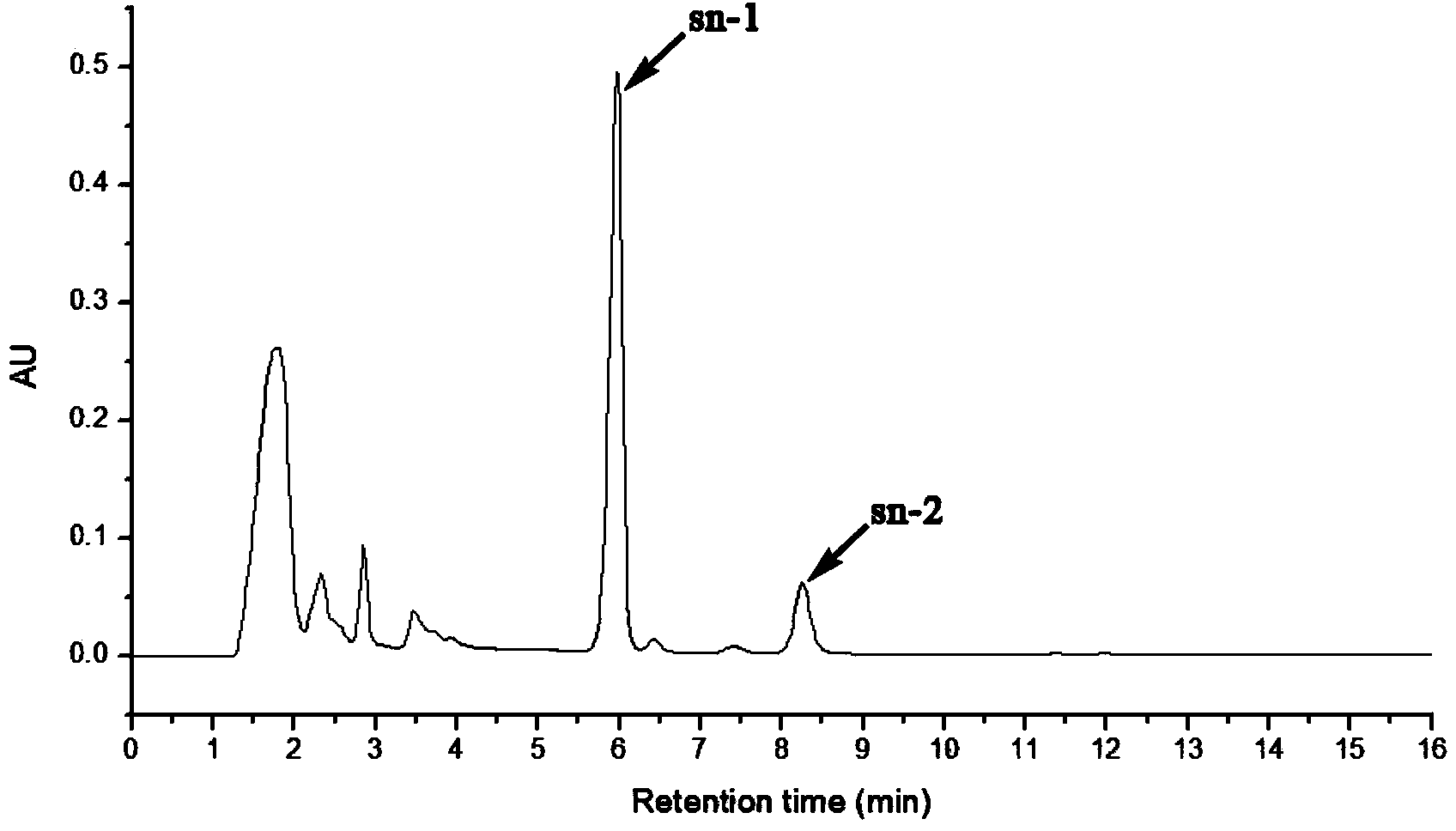
Selective synthesis method of sn-1 chloropropanol fatty acid ester
A fatty acid ester, sn-1 technology, applied in the field of selective synthesis of sn-1 chloropropanol fatty acid ester, can solve the problems of difficult removal of catalysts, limited types, high price, etc., and achieves easy acquisition, less use, low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Add 10g molecular sieve (SiO 2 :Al 2 o 3 =50:1), magnetically stirred for 5 hours in a water bath at 70°C, suction filtered while hot, and vacuum-dried at 120°C for 2 hours to prepare the ferric sulfate catalyst supported by aluminosilicate molecular sieves. Then take by weighing 0.05mol of lauric acid and place it in a 100mL round-bottomed flask, preheat and melt in a water bath of 60° C. 5% of the total weight of epichlorohydrin) was stirred and mixed, and then 0.05mol of epichlorohydrin was added dropwise. After 24 hours, the reaction was stopped, the product was filtered with suction to remove the catalyst, washed with methanol, and the solvent was evaporated to obtain sn-1 chloropropanol monolaurate.
Embodiment 2
[0024] In 0.2mol / L ferric chloride aqueous solution (50mL), add 10g molecular sieve (SiO 2 :Al 2 o 3 =80:1), stirred magnetically for 4 hours in a water bath at 80°C, filtered while hot, and dried at 120°C for 2 hours to prepare a molecular sieve-supported ferric chloride catalyst. Then weigh 0.05mol of n-decanoic acid and place it in a 100mL round-bottomed flask, preheat and melt it in a water bath at 50° C. 2% of the total weight of capric acid and epichlorohydrin) was stirred and mixed, and then 0.025mol of epichlorohydrin was added dropwise. After 40 hours, the reaction was stopped, the product was filtered with suction to remove the catalyst, washed with methanol, and the solvent was evaporated to obtain sn-1 chloropropanol n-decanoic acid monoester.
Embodiment 3
[0026] In 0.2mol / L ferric nitrate aqueous solution (50mL), add 10g molecular sieve (SiO 2 :Al 2 o 3 =38:1), magnetically stirred for 4 hours in a water bath at 90°C, suction filtered while hot, and dried at 120°C for 2 hours to prepare a molecular sieve-supported ferric nitrate catalyst. Then take by weighing the n-octanoic acid of 0.05mol and place in the 100mL round-bottomed flask, preheat and melt in the water bath pan of 50 ℃ and the ferric nitrate catalyst (being that catalyst amount is reactant n-octanoic acid and 3% of the total weight of epichlorohydrin) was stirred and mixed, and then 0.10mol of epichlorohydrin was added dropwise. The reaction was stopped after 60 hours, the product was filtered with suction to remove the catalyst, washed with methanol, and the solvent was evaporated to obtain sn-1 chloropropanol n-octanoic acid monoester.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com