Lithium vanadate negative electrode material of lithium ion battery and preparation method thereof
A battery lithium vanadate, lithium ion battery technology, applied in battery electrodes, secondary batteries, chemical instruments and methods, etc., can solve the problem that the role of negative electrode materials has not been discovered, lithium vanadate has not been studied, and the development of lithium titanate has been limited. and other problems, to achieve the effect of improving electrochemical performance, improving electrical conductivity, and simple preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Will Li 2 CO 3 and V 2 o 5 Weigh according to the stoichiometric ratio of Li:V =3:1 (Li 2 CO 3 : 3.231g, V 2 o 5 : 2.624g), using a planetary ball mill for 4 hours;
[0042] Then, under the air atmosphere, heat to 350°C for pretreatment for 5 hours, and then grind again after cooling in the furnace;
[0043] Then, in the air atmosphere, sintering at a temperature of 750° C. for 10 hours to obtain a lithium vanadate negative electrode material for a lithium ion battery.
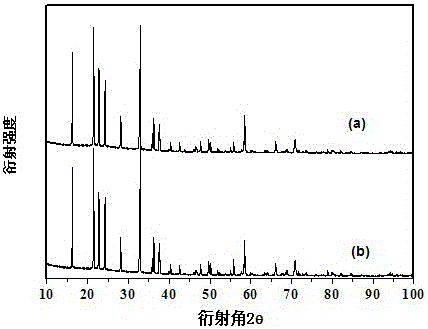
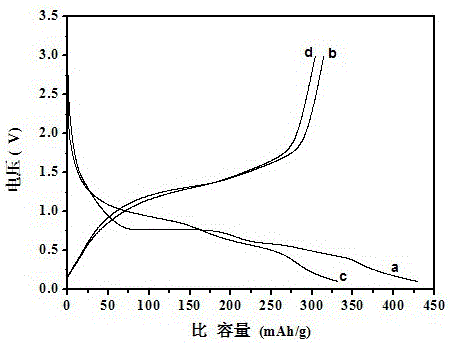
[0044] The XRD pattern of the product is shown in figure 1 From the curve in (a), it can be seen from the figure that a pure-phase lithium vanadate negative electrode material is synthesized by using this solid-state sintering method. There is no impurity peak in the spectrum, and the product has high purity. . The lithium vanadate negative electrode material is in the voltage range of 0.1-3.0V, and the rate is 0.2C. The first and second charge and discharge curves are as follows: figure 2 s...
Embodiment 2
[0046] Combine LiOH and NH 4 VO 3 Weigh according to the stoichiometric ratio of Li:V=3:1 (LiOH:2.518g, V 2 o 5 : 2.340g), using a planetary ball mill for 4 hours;
[0047] Then at 30%H 2 + 70% Ar (volume fraction ratio) atmosphere, heated to 350°C for pretreatment for 5 hours, and then ground again after cooling in the furnace;
[0048] Then at 30%H 2 + 70% Ar (volume fraction ratio) atmosphere, sintering at a temperature of 700 ° C for 10 hours to obtain a lithium vanadate negative electrode material for a lithium ion battery.
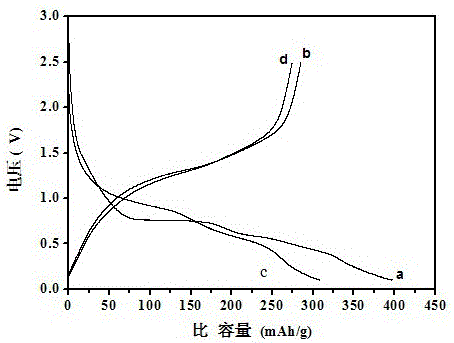
[0049] The XRD pattern of the product is shown in figure 1 From the curve in (b), it can be seen from the figure that a pure-phase lithium vanadate negative electrode material is synthesized by using this solid-state sintering method. There is no impurity peak in the spectrum, and the product has high purity. The lithium vanadate negative electrode material is in the voltage range of 0.1-2.5V, and the rate is 0.2C. The first and second charg...
Embodiment 3
[0051] CH 3 COOLi and V 2 o 5 Weigh according to the stoichiometric ratio of Li:V =3:1 (CH 3 COOLi: 3.06g, V 2 o 5 : 1.819g), using a planetary ball mill for 4 hours;
[0052] Then, under the air atmosphere, heat to 350°C for pretreatment for 5 hours, and then grind again after cooling in the furnace;
[0053] Then in 70%Ar+30H 2 % (volume fraction ratio) atmosphere, sintering at 750 ° C for 10 h to obtain lithium vanadate negative electrode materials for lithium-ion batteries.
[0054] The XRD pattern of the product is shown in Figure 5 From the curve in (a), it can be seen from the figure that a pure-phase lithium vanadate negative electrode material is synthesized by using this solid-state sintering method. There is no impurity peak in the spectrum, and the product has high purity.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com