Lithium nickel cobalt manganate hollow sphere as well as preparation method and application thereof
A technology of nickel cobalt lithium manganate and hollow spheres, applied in chemical instruments and methods, manganate/permanganate, nickel compounds, etc., can solve the problems of low specific capacity and rate performance of materials, and improve specific capacity. and rate performance, more active sites for lithium storage, and the effect of large specific surface area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
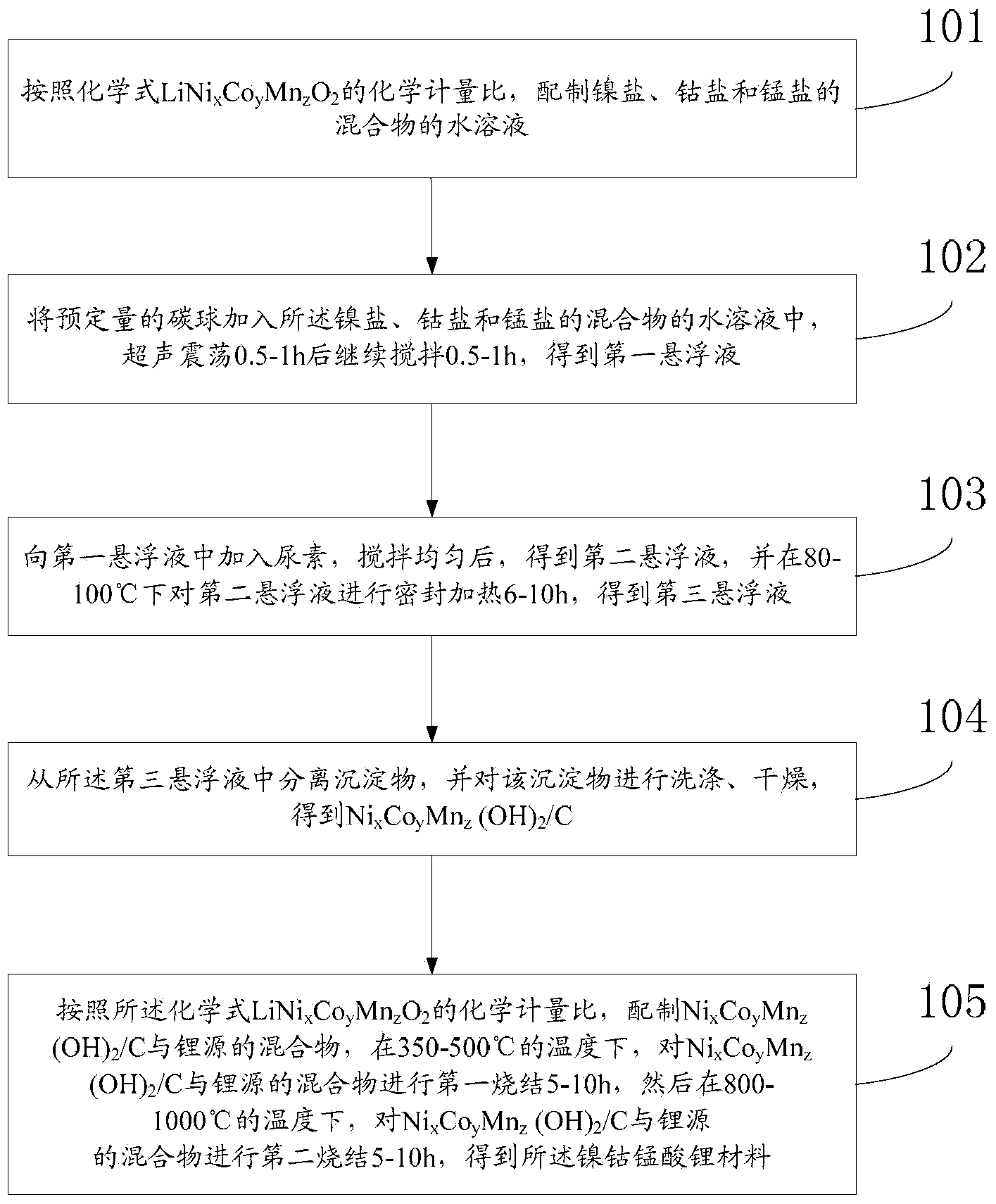
[0043] In the third aspect, the embodiment of the present invention provides a method for preparing nickel-cobalt-lithium-manganese-oxide hollow spheres, with figure 1 For the preparation flow chart of this method, as attached figure 1 As shown, the method includes:
[0044] Step 101, according to the chemical formula LiNi x co y mn z o 2 The stoichiometric ratio, the preparation of the aqueous solution of the mixture of nickel salt, cobalt salt and manganese salt.
[0045] Wherein, nickel salt is selected from at least one of nickel nitrate, nickel sulfate, nickel chloride; Cobalt salt is selected from at least one of cobalt nitrate, cobalt sulfate, cobalt chloride; Manganese salt is selected from manganese nitrate, manganese sulfate, At least one of manganese chloride.
[0046] Step 102, adding a predetermined amount of carbon spheres into the aqueous solution of the mixture of nickel salt, cobalt salt and manganese salt, ultrasonically oscillating for 0.5-1 h and then...
Embodiment 1
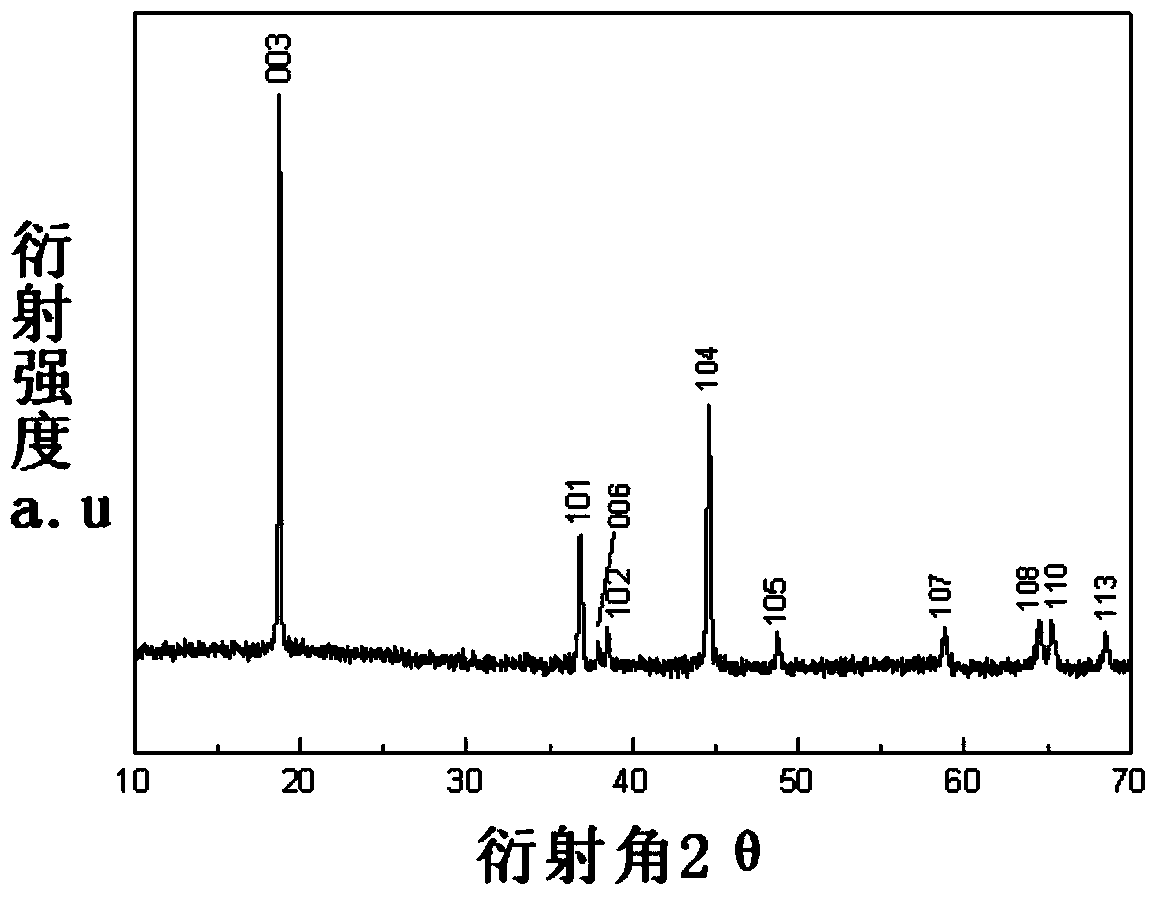
[0063] Prepare 100 mL of an aqueous solution of a mixture of nickel nitrate, cobalt nitrate, and manganese nitrate with a concentration of 1 mol / L, and add 0.2 g of carbon spheres to it, then ultrasonically vibrate for 0.5 h, and then continue to stir for 0.5 h. A first suspension is obtained. Add 0.35 mol of urea to the first suspension, stir well to obtain a second suspension, transfer the second suspension to a flask, heat to 80°C after sealing well and keep it warm for 10 hours to obtain a third suspension . After the third suspension was naturally cooled to room temperature, it was centrifuged to obtain a precipitate, which was washed several times with distilled water and ethanol, and then dried at 60°C to obtain Ni 1 / 3 co 1 / 3 mn 1 / 3 (OH) 2 / C.
[0064] According to the molar ratio Ni+Mn+Co:Li=1:1.1, the Ni 1 / 3 co 1 / 3 mn 1 / 3 (OH) 2 Add / C and lithium hydroxide to 20mL ethanol solution, stir until the ethanol solution is completely evaporated to obtain a solid mi...
Embodiment 2
[0067] Prepare 100 mL of an aqueous solution of a mixture of nickel sulfate with a concentration of 2 mol / L, cobalt sulfate with a concentration of 1 mol / L, and nickel-cobalt-manganese salt with a concentration of 2 mol / L manganese sulfate, and add 0.4 g of carbon spheres to it, and then ultrasonically After shaking for 1 h and continuing to stir for 1 h, the first suspension was obtained. Add 0.55 mol of urea to the first suspension, stir well to obtain the second suspension, transfer the second suspension to a flask, heat to 100°C after sealing well and keep it warm for 6 hours to obtain the third suspension . After the third suspension was naturally cooled to room temperature, it was centrifuged to obtain a precipitate, which was washed several times with distilled water and ethanol, and then dried at 60°C to obtain Ni 0.4 co 0.2 mn 0.4 (OH) 2 / C.
[0068] According to the molar ratio Ni+Mn+Co:Li=1:1.1, the Ni 0.4 co 0.2 mn 0.4 (OH) 2 / C and lithium carbonate are a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com