Drug composition containing domperidone
A technology for domperidone and medicines, applied in the field of pharmaceutical preparations, can solve the problems of the combined oral dosage forms of domperidone and ilaprazole sodium that have not yet been reported, and achieve the effects of being convenient for long-term storage, easy to use, and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
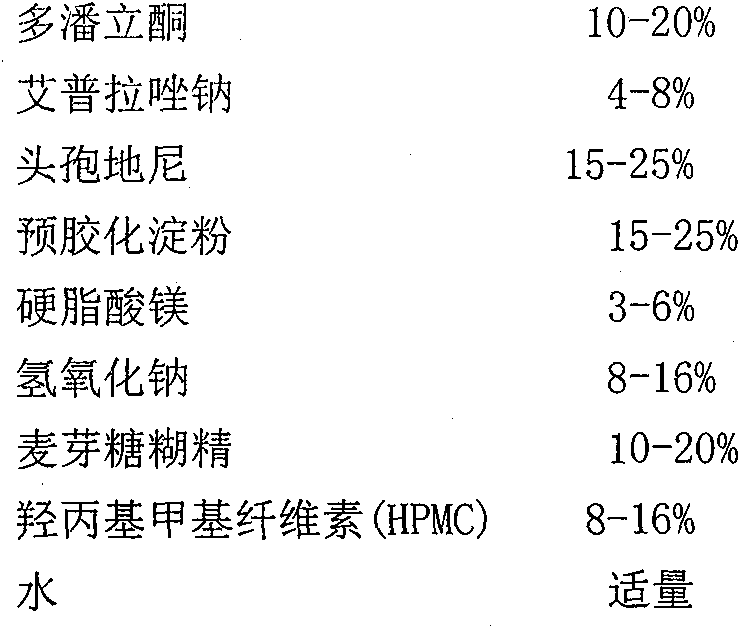
[0020] (1) Drug core prescription
[0021]
[0022] Drug core preparation process: (1) Weigh half of the prescribed amount of cefdinir and pregelatinized starch, and mix through a 60-mesh sieve; weigh the prescribed amount of ilaprazole sodium and sodium hydroxide, and mix through a 60-mesh sieve sieve; mix the above two;
[0023] (2) Take by weighing half of the cefdinir of the prescription quantity, domperidone and maltodextrin of the prescription quantity, and mix through a 60 mesh sieve;
[0024] (3) Weigh 5 cp of hydroxypropyl methylcellulose (HPMC) in the prescribed amount, disperse with a small amount of hot water, add an appropriate amount of water to dissolve, and make pulp (8% (m / m)). The above-mentioned mixed material, magnesium stearate and slurry are made into a soft material, and the soft material is extruded and spheronized.
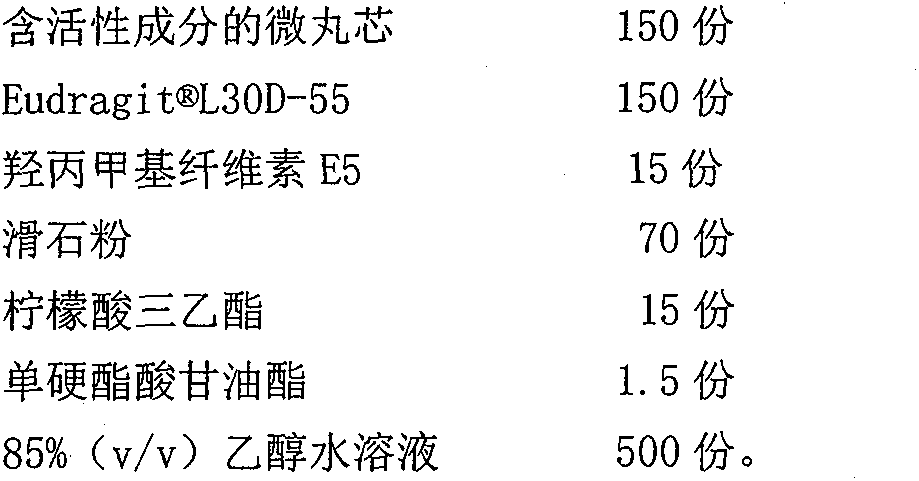
[0025] (2) Enteric coating
[0026]
[0027] Enteric coating layer preparation method: add 4% (m / v) sodium hydroxide aqueous sol...
Embodiment 2
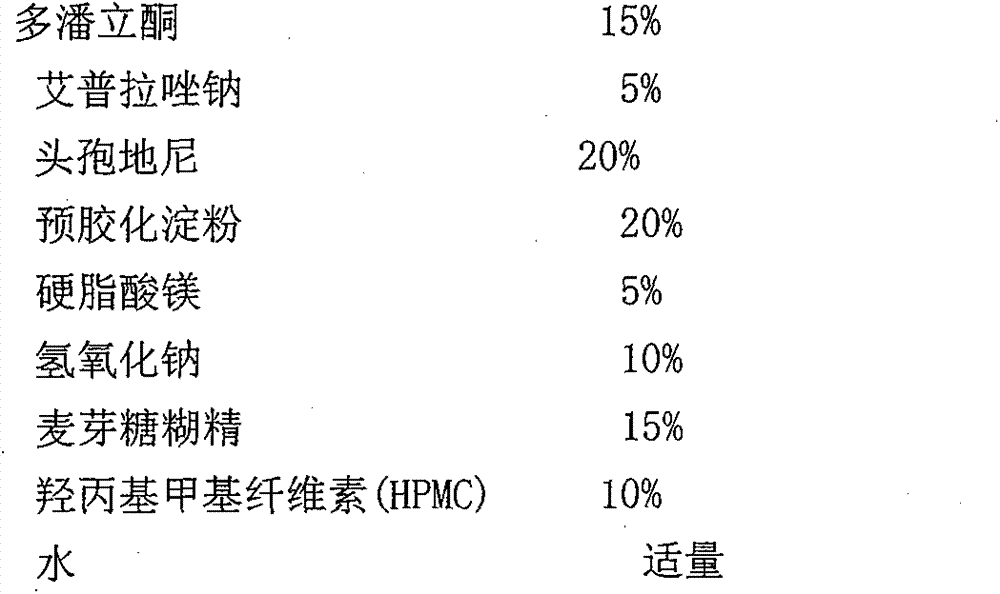
[0029] (3) Drug core prescription
[0030]
[0031]
[0032] Drug core preparation process: (1) Weigh half of the prescribed amount of cefdinir and pregelatinized starch, and mix through a 60-mesh sieve; weigh the prescribed amount of ilaprazole sodium and sodium hydroxide, and mix through a 60-mesh sieve sieve; mix the above two;
[0033] (2) Take by weighing half of the cefdinir of the prescription quantity, domperidone and maltodextrin of the prescription quantity, and mix through a 60 mesh sieve;
[0034] (3) Weigh 5 cp of hydroxypropyl methylcellulose (HPMC) in the prescribed amount, disperse with a small amount of hot water, add an appropriate amount of water to dissolve, and make pulp (8% (m / m)). The above-mentioned mixed material, magnesium stearate and slurry are made into a soft material, and the soft material is extruded and spheronized.
[0035] (4) Enteric coating
[0036]
[0037] Enteric coating layer preparation method: add 4% (m / v) sodium hydroxide...
Embodiment 3
[0039] Embodiment 3: The optimum ratio screening test of ilaprazole and domperidone in the combination medicine of the present invention
[0040] In the comparative experiment, the active ingredients in the drug core of the present invention: domperidone and ilaprazole sodium were prepared into preparations of different specifications in different proportions, and the single PPI or H2-receptor antagonist was orally treated twice a day. For gastric ulcer and duodenal ulcer that cannot be effectively treated, the treatment effect data are as follows:
[0041] Table 1: Screening test for optimal ratio of ilaprazole and domperidone
[0042] Specification The overall cure rate of gastric ulcer Overall cure rate of duodenal ulcer Ilaprazole Sodium 150g / Domperidone 50g 70.2% 71.5% Ilaprazole Sodium 150g / Domperidone 100g 72.3% 72.6% Ilaprazole Sodium 150g / Domperidone 150g 74.0% 73.0% Ilaprazole Sodium 50g / Domperidone 100g 80.1% 82.9% I...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com