Gene chip and kit for detecting swine epidemic encephalitis B viruses (SEEBVs) and/or swine fever viruses (SFVs)
A technology of Japanese encephalitis virus and swine fever virus, which is applied in the fields of biochemical equipment and methods, measurement/testing of microorganisms, nucleotide library, etc., can solve the problem of sensitive detection of Type B without chip sensitivity test Encephalitis virus and swine fever virus and other problems have achieved good application prospects, rapid detection, and short time-consuming effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Example 1 Preparation of the gene chip of the present invention
[0049] 1. Materials and instruments
[0050] The same experimental materials and instruments as mentioned above.
[0051] 2. Experimental method
[0052] 2.1 Preparation of PCR primers and detection probes
[0053] (1) Design of pathogen-specific probes: through the alignment analysis of the nucleic acid sequences of porcine epidemic encephalitis virus and classical swine fever virus included in GenBnak, the sequences of conserved regions were selected: JEV C, M, E, NS1, NS5; CSFV 5'UTR, 3'UTR, Npro, Erns, E2. Design detection probes for conserved sequences, and select specific probe sequences from them.
[0054] (2) Designing specific primers for the probe sequences: specific primers were designed using the bioinformatics software Primer5 for the above conserved probe sequences. Specific primers were synthesized by Dalian Bao Biological Company.
[0055] (3) Probe preparation: use a small amount of v...
Embodiment 2
[0162] Embodiment 2 Detection method of the present invention
[0163] 1. Nucleic acid extraction
[0164] Tiangen’s Total RNA Extraction Kit, refer to the instructions for operation, the specific steps are as follows:
[0165] 1) Use scissors to cut tissue disease material of appropriate size into a mortar, add an appropriate amount of RZ lysate, grind until homogenized, take 300μl in a 1.5ml EP tube, add 1ml of RZ lyse solution for every 50-100mg of tissue, the sample volume is not It should be more than one-tenth of the RZ volume of the lysate.
[0166] 2) Place the homogenized sample at 15-30°C for 5 minutes;
[0167] 3) Centrifuge at 12000r / min for 5min at 4°C, take the supernatant, and transfer it to a new RNase-free centrifuge tube;
[0168] 4) Add 200 μL of chloroform, shake vigorously up and down for 15 sec, and let stand at room temperature for 3 min;
[0169] 5) Centrifuge at 12000r / min for 10min at 4°C, the sample will be divided into three layers, and gently t...
Embodiment 3
[0199] Embodiment 3 specificity test
[0200] 1. Test method
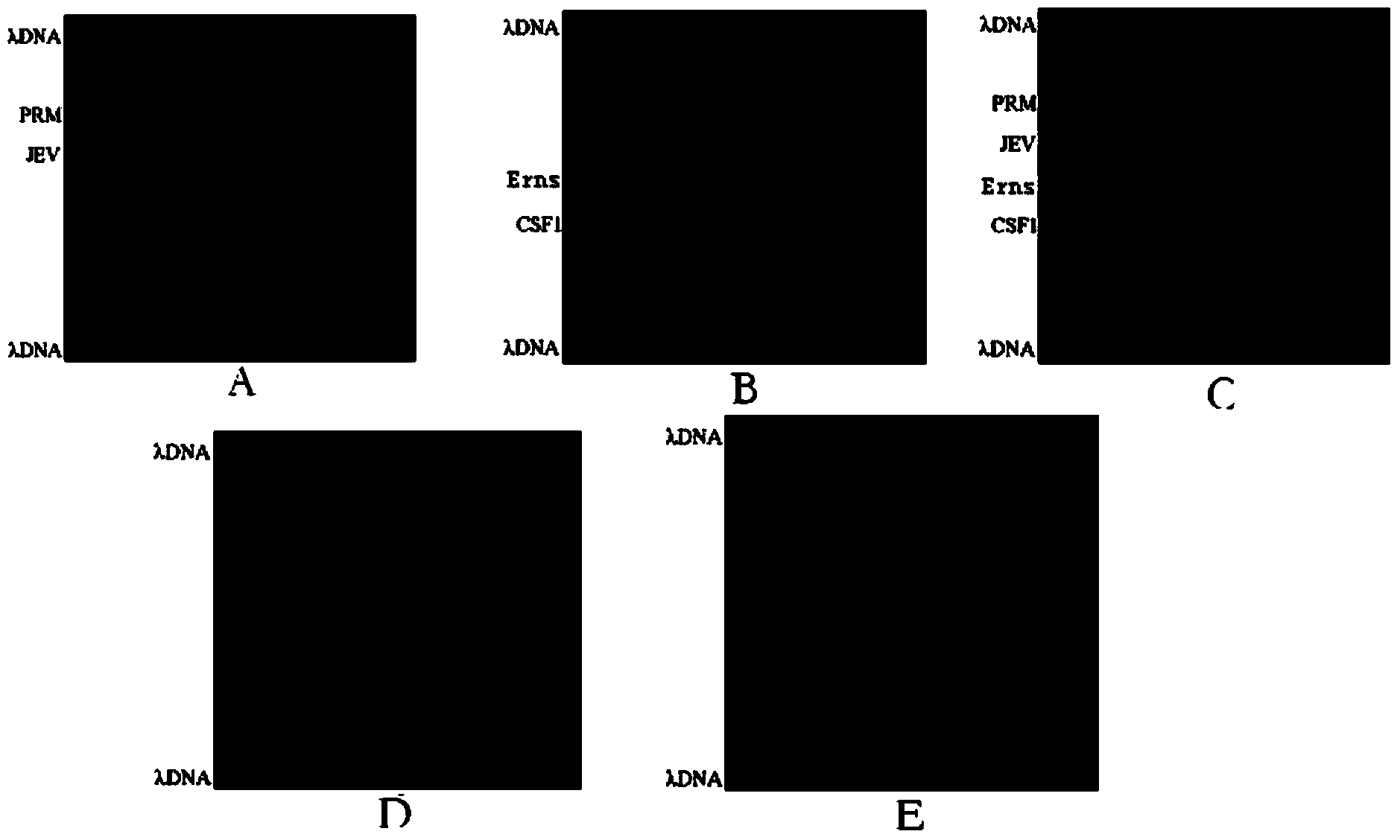
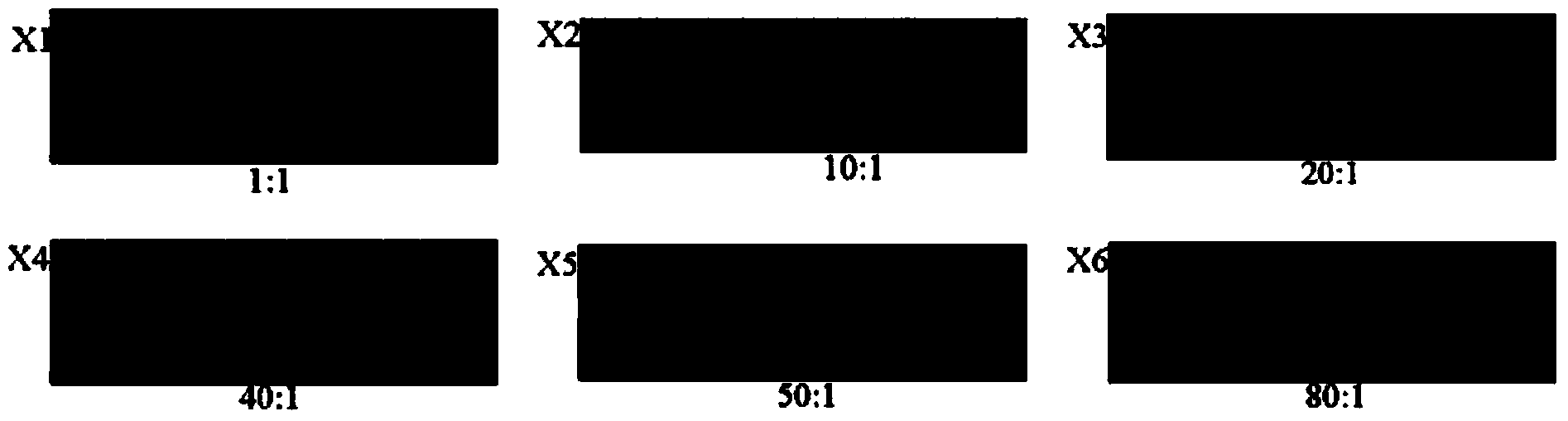
[0201] Using the gene chip prepared in Example 1, according to the method of Example 2 (the reverse primer of Prm / M, JEV-C, Erns, CSF1 and the forward marker primer molar ratio are 50:1, 40:1, 80:1, 80:1, the hybridization temperature is 48°C, and the hybridization time is 3h), and JEV, CSFV, PPV, and PCV-2 are detected to verify the specificity of the gene chip and the kit of the present invention.
[0202] 2. Results
[0203] Experimental results such as Figure 6 As shown, the method of the present invention can effectively detect CSFV and JEV separately or simultaneously, but will not detect other viruses, such as PPV and PCV-2.
[0204] The experimental results show that the specificity of the kit and the gene chip of the present invention is strong, and other viruses cannot be detected.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com