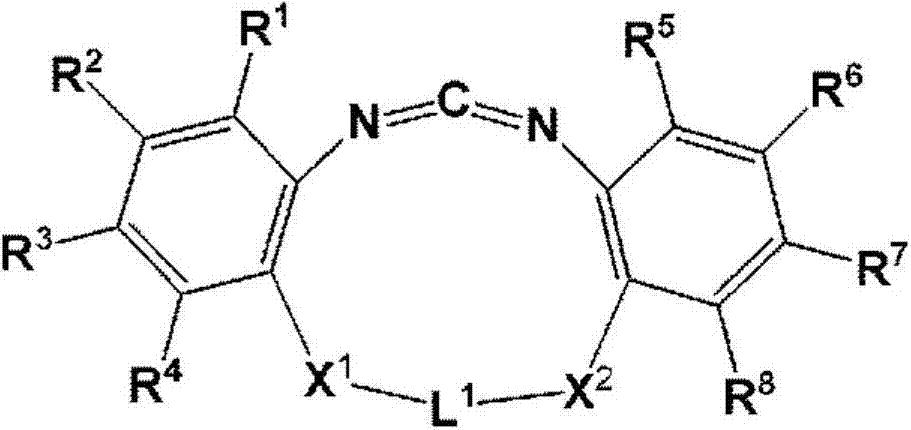
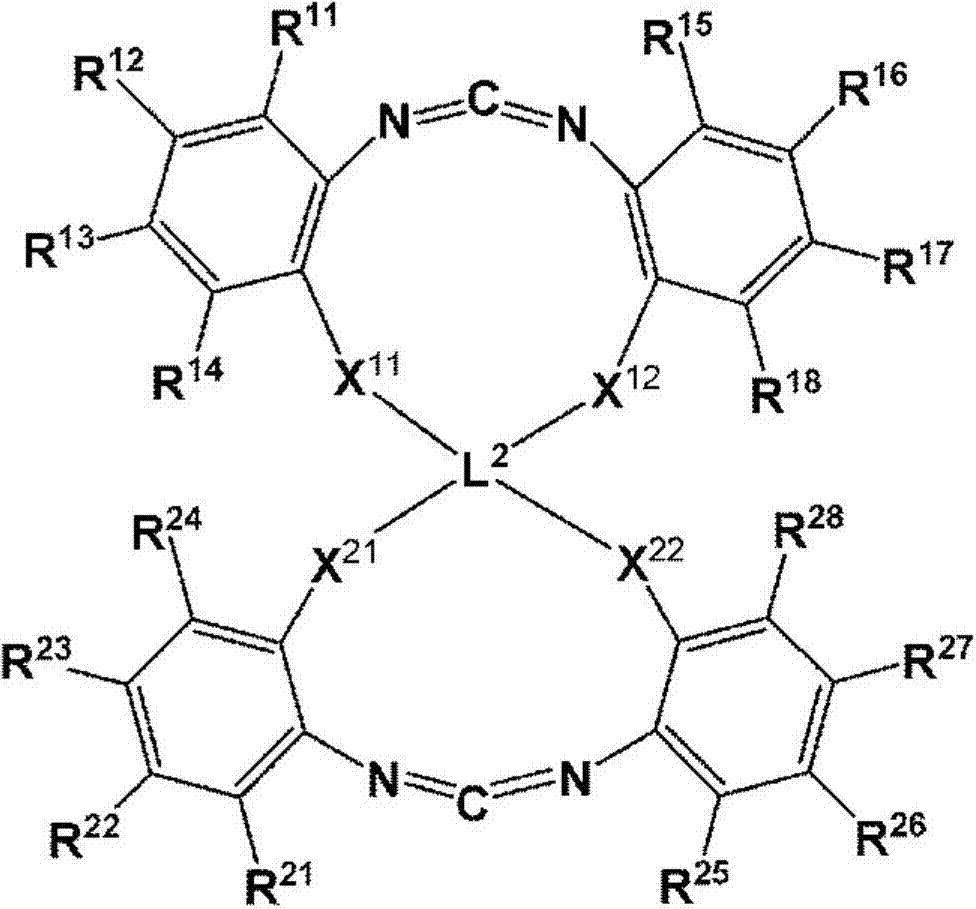
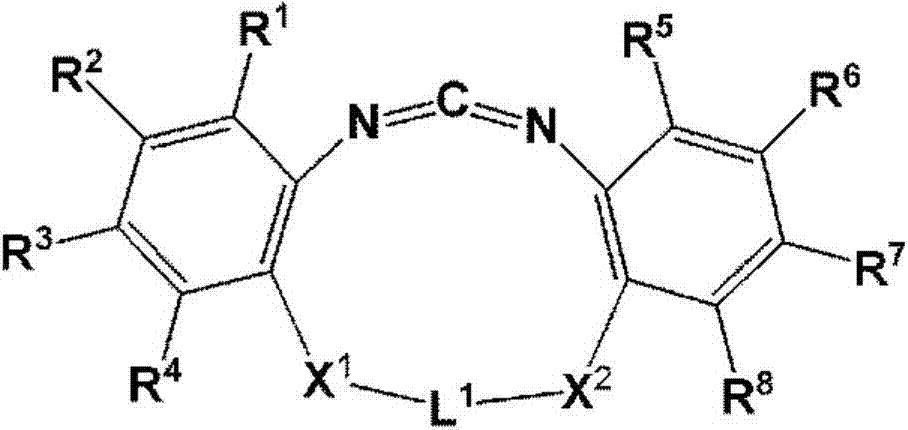
Cyclic carbodiimide compound, polyester film, back sheet for solar cell module and solar cell module
A carbodiimide and compound technology, applied in the field of solar cell modules, can solve the problems of small molecular weight, irritating gas volatilization, etc., and achieve the effect of inhibiting viscosity increase, inhibiting generation, and good film thickness uniformity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0242] Examples are listed below to further specifically illustrate the present invention. The materials, usage amounts, ratios, processing contents, processing procedures, etc. shown in the following examples can be appropriately changed as long as they do not depart from the gist of the present invention. Therefore, the scope of the present invention is not limited to the examples shown below. In addition, unless otherwise specified, "parts" is a quality standard.
[0243] As the carbodiimide-based end-capping agent, the following compounds were used in each comparative example. In addition, the cyclic carbodiimide (1) used in the comparative example is a compound with a molecular weight of 252 described in the examples of JP 2011-258641 A, please refer to the reference example of JP 2011-258641 Synthesize by the synthesis method described in 1.
[0244] The cyclic carbodiimide (2) used in the comparative example is a compound with a molecular weight of 516 described in the Ex...
Synthetic example 1
[0254] (Synthesis of compound 1)
[0255] In a reaction device equipped with a stirring device, a mixture of nitric acid (1.1mol) and 800ml of acetic acid was slowly added dropwise to carvacrol (1.0mol) and 1.6L of acetic acid while stirring, and then extracted with 1L of ethyl acetate. And wash thoroughly. After the obtained organic layer was dehydrated with magnesium sulfate, it was concentrated. After that, it was purified by column chromatography to obtain 50 g of carvacrol's o-nitro body.
[0256] Then put the nitro body (0.1mol) obtained above and 1,2-dibromoethane (0.05mol), potassium carbonate (0.3mol), 200ml of N,N-dimethylformamide in N 2 Put it into a reaction device equipped with a stirring device and a heating device under an atmosphere. After reacting at 130°C for 12 hours, remove DMF under reduced pressure, and dissolve the resulting solid matter in 200ml of dichloromethane, and perform 3 times with 100ml of water Liquid separation. The organic layer was dehydrate...
Synthetic example 2
[0264] (Synthesis of Compound 2)
[0265] Put the nitro body (0.1mol) obtained in Synthesis Example 1 with tetrabromopentaerythritol (0.025mol), potassium carbonate (0.3mol), and 200ml of N,N-dimethylformamide in N 2 Put it into a reaction device equipped with a stirring device and a heating device under an atmosphere. After reacting at 130°C for 30 hours, the DMF is removed under reduced pressure, and the resulting solid substance is washed with water, ethanol, and hexane to obtain Intermediate A (Nitro body).
[0266] Then, intermediate product A (0.02 mol), 560 ml of 2-isopropanol and 110 ml of 35% hydrochloric acid aqueous solution were put into a reaction vessel equipped with a stirring device, and zinc powder (0.8 mol) was slowly added, and then refluxed for 1 hour. After the zinc powder was recovered by filtration, 1.0 L of chloroform was added, and liquid separation was performed twice with pure water. The organic layer was dehydrated with magnesium sulfate, and chloroform...
PUM
| Property | Measurement | Unit |
|---|---|---|
| heat shrinkage ratio | aaaaa | aaaaa |
| viscosity index | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com