Gremlin-1 antibody
A technology of antibodies and growth factor receptors, applied in the direction of antibodies, anti-inflammatory agents, anti-tumor drugs, etc., can solve the problems that the effects have not been studied in detail, and achieve the effect of preventing or treating cancer or immune diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Example 1: Expression and purification of Gremlin-1
[0067] 1-1: Cell culture
[0068] A549, HeLa, A172 and A431 cells were obtained from the Korean Cell Line Bank (Seoul, Korea), and human umbilical vein endothelial cells (HUVEC) were obtained from Invitrogen (Carlsbad, CA). Meanwhile, A549, A172 and A431 cells were cultured in RPMI-1640 medium (Welgene) supplemented with 10% FBS, and HeLa cells were cultured in MEM medium (Welgene) supplemented with 10% FBS, while HUVEC endothelial cells Cultured in growth medium-2 (EGM-2, Lonza, Walkersville, MD).
[0069] 1-2: Preparation of Gremlin-1 expression vector and cell transfection
[0070] Gremlin cDNA was amplified from a human cervical tissue cDNA library as described in Namkoong H et al. (2006) BMC Cancer 6:74. Next, HindIII and XhoI restriction enzyme sites were introduced into the 5' and 3' ends of the gremlin cDNA by PCR using the following PCR primers:
[0071] 5'-CCC AAG CTT ATG AGC CGC ACA GCC TAC AC-3'...
Embodiment 2
[0086] Example 2: Generation of Gremlin-1 Antibody
[0087] 2-1: Immunization
[0088] 5 μg of gremlin-1-Fc was mixed with 2 mL of phosphate-buffered saline (PBS) and incubated at 37° C. for 30 minutes, and the mixture was dissolved in 2% squalene containing detoxified endotoxin MPL (monophosphorylated lipid A species) and mycobacterial cell wall components (TDW and CWS) in an oil-in-water emulsion adjuvant (Sigma, St. Louis, Mo) and injected into New Zealand white rabbits . Immunization was performed three times at 3-week intervals. Antibody titers of immunized rabbits were determined by enzyme-linked immunosorbent assay (ELISA) using horseradish peroxidase (HRP)-conjugated mouse anti-rabbit IgG polyclonal antibody (Pierce Chemical Co., Rockford, IL). ) as the secondary antibody.
[0089] Total RNA was obtained from spleen and bone marrow of immunized rabbits using TRI reagent (Invitrogen). Extracted spleen and bone marrow were homogenized in TRI reagent at 50% outpu...
Embodiment 3
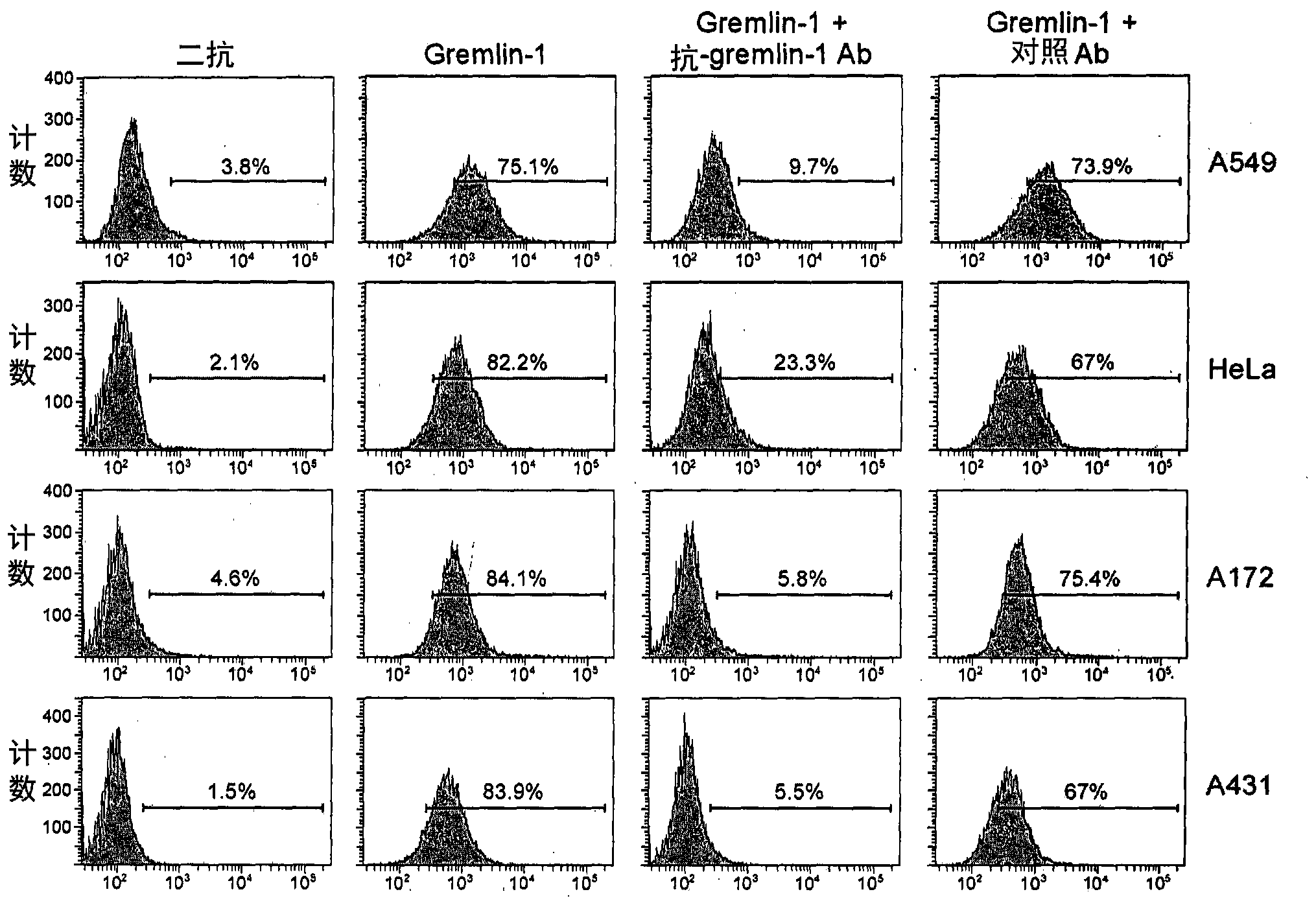
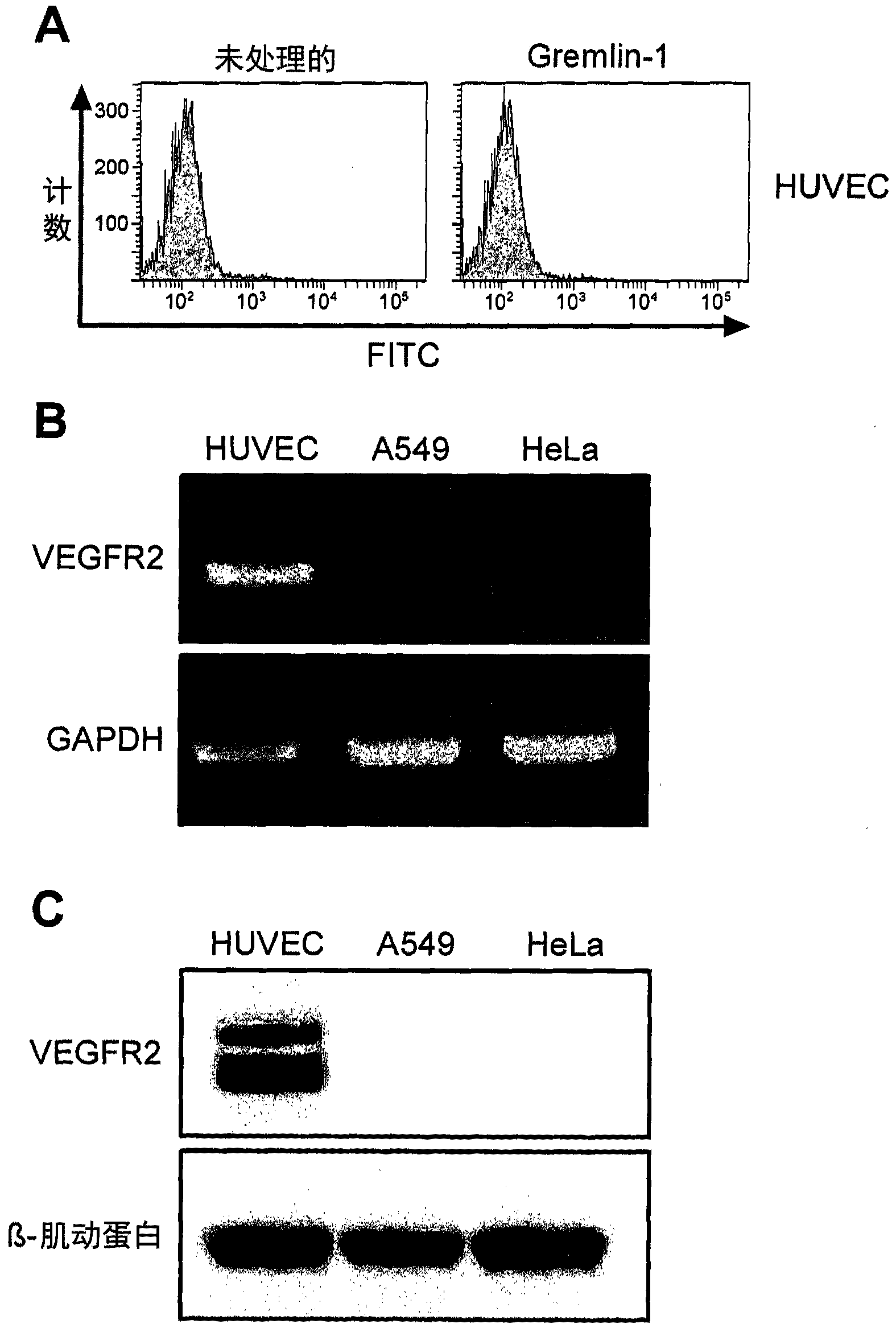
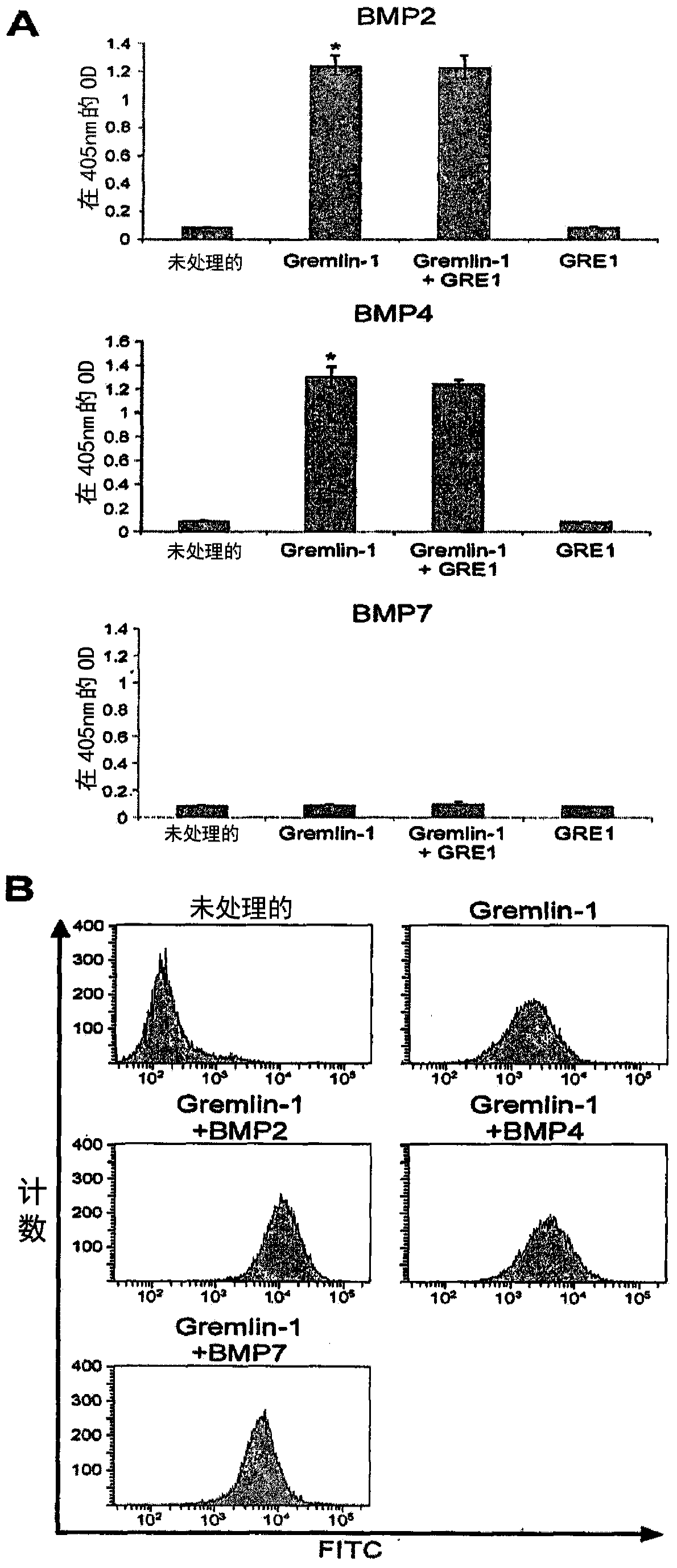
[0114] Example 3: Analysis of the interaction between Gremlin-1 and cancer cells
[0115] To examine whether gremlin-1 directly interacts with cancer cells, gremlin-1 was incubated with four cancer cells (A549, HeLa, A172 and A431) and then analyzed by flow cytometry.
[0116] Specifically, adherent cells were trypsinized and washed with 1% (w / v) BSA in phosphate buffered saline (PBS). Suspension cells were harvested by centrifugation at 500 xg for 2 minutes and washed with 1% (w / v) BSA in PBS. All cells were incubated with His-tagged gremlin-1 (R&D Systems, Minneapolis, MN) at a final concentration of 100 nM in 1% (w / v) BSA in PBS. Cells were then washed twice with 1% (w / v) BSA in PBS and incubated with FITC-conjugated His antibody (Abcam, Cambridge, UK) at a final concentration of 5 mg / ml at 37°C for 30 minutes in the dark. Cells were then washed twice with 1% (w / v) BSA in PBS and resuspended in 500 μL of PBS before analysis on a FACSCanto II flow cytometer (BD Bioscienc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com