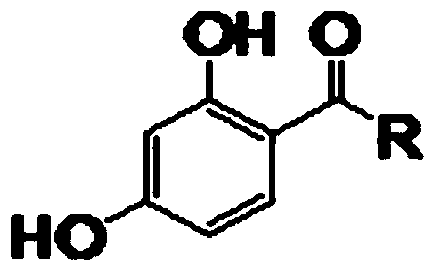
Preparation method of 1, 4-dihydroxy-phenyl ketone
A technology of phenyl ketone and dihydroxyl, which is applied in the field of preparation of 1,4-dihydroxy-phenyl ketone, can solve the problems of only 60% conversion rate, high operation requirements, unreacted by-product resorcinol and the like , to achieve the effect of effective recycling and utilization, simple process and easy recycling
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Add 9.9g of n-butyric acid, 21mL of toluene, and 10g of anhydrous zinc chloride into a 50mL flask, immerse in a preheated 100°C oil bath, stir to a constant temperature, add 6.05g of resorcinol, and stir for 30min. Raise the temperature to 110°C, continue the reaction for 5 hours, cool to 0-5°C, separate the lower layer of zinc chloride (with n-butyric acid, a small amount of resorcinol and products), and wash the toluene layer with 6M hydrochloric acid 1-2 times, 10 % sodium hydroxide (20mL*5) was fully extracted, the extracted water phase was adjusted to a pH value of about 3.0 with concentrated hydrochloric acid, a large amount of solids were precipitated, cooled to 5-10°C, suction filtered, washed with water, recrystallized with 37.5% ethanol aqueous solution, 7.8 g of yellow needle-like crystals were obtained, and the product yield was 79% (calculated by resorcinol).
Embodiment 2
[0030] Add 10.45g of n-propionic acid, 32mL of cyclohexane, 6.05g of resorcinol, and 10g of anhydrous zinc chloride into a 100mL flask, immerse in a preheated oil bath, reflux with water for 6 hours, and cool to 0 ~5°C, separate the lower layer of zinc chloride (with n-propionic acid, a small amount of resorcinol and products), wash the organic layer with 6M hydrochloric acid for 1 to 2 times, and fully extract with 10% sodium hydroxide (20mL*5) Afterwards, extract the aqueous phase and use concentrated hydrochloric acid to adjust the pH value to about 3.0, a large amount of solids are precipitated, cooled to 5-10 ° C, filtered with suction, washed with water, and recrystallized with 35% ethanol aqueous solution to obtain 7.1 g of yellow needle-shaped crystals. The product yield is 77%.
Embodiment 3
[0032] Add 9.74g of n-hexanoic acid, 35mL of xylene, and 10g of anhydrous zinc chloride into a 100mL flask, immerse in a preheated 100°C oil bath, stir to a constant temperature, add 6.05g of resorcinol, and stir for 30min. Raise the temperature to 120°C, continue the reaction for 5.5 hours, cool to 0-5°C, separate the lower layer of zinc chloride (containing n-hexanoic acid, a small amount of resorcinol and products), and wash the xylene layer with 6M hydrochloric acid for 1-2 times. Fully extract with 10% sodium hydroxide (20mL*5), adjust the pH value of the extracted water phase to about 3 with concentrated hydrochloric acid, precipitate a large amount of solid, cool to 5-10°C, filter with suction, wash with water, recrystallize with 39% ethanol aqueous solution, 8.9 g of yellow needle-like crystals were obtained, and the product yield was 76%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com