N-containing biheterocyclic acylamide compounds as well as application thereof as immunosuppressor
A technology of heterocyclic amides and compounds, applied in the field of medical immunology, can solve problems such as poor targeting, and achieve simple preparation methods, small molecular weight, and significant application effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
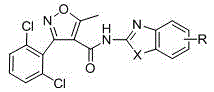
[0041] Example 1: Preparation of 3-(2,6-dichlorophenyl)-5-methyl-N-2-(6-methylsulfonylbenzothiazolyl)isoxazole-4-carboxamide :
[0042] Under anhydrous and oxygen-free conditions (argon protection), 0.347 g (1.2 mmol) of 3-(2,6-dichlorophenyl)-5-methylisoxazole-4-carbonyl chloride (product 2) was dissolved in 4 mL of anhydrous dichloromethane and place it in a pre-dried constant pressure funnel. Add 0.212 g (1 mmol) of 2-amino-6-thiamphenicol benzothiazole to a dry 50 mL three-neck round bottom flask, and add 5 mL of anhydrous dichloromethane and 1 mL of anhydrous N,N-di Methylformamide (DMF) was dissolved as a reaction solubilizer, and 0.25 mL of anhydrous triethylamine was used as an acid-binding agent. Add dropwise 4 mL of dichloromethane solution in which 3-(2,6-dichlorophenyl)-5-methylisoxazole-4-carbonyl chloride was dissolved in a round-bottomed flask placed in a low-temperature reactor . Keep the temperature at 40~45 o C. After the dropwise addition was completed...
Embodiment 2
[0043] Example 2: Preparation of 3-(2,6-dichlorophenyl)-5-methyl-N-[2-(6-ethylbenzothiazole)isoxazole]-4-formamide:
[0044] Under anhydrous and oxygen-free conditions (argon protection), take 0.178 g (1 mmol) of 2-amino-6-ethylbenzothiazole in a three-necked round-bottomed flask, add 5 mL of anhydrous dichloroethane and 1 mL of anhydrous DMF was used as the reaction solvent, 0.25 ml of anhydrous triethylamine was used as an acid-binding agent, mixed at low temperature and cooled to 0 o C, and keep for 15 minutes. A dichloromethane solution of 0.347 g (1.2 mmol) of 3-(2,6-dichlorophenyl)-5-methylisoxazole-4-carbonyl chloride was added dropwise to the reaction system. After stirring at low temperature for 30 minutes, transfer to the oil bath 60 o C was reacted for 18 hours. The solution turned from yellow to tan. The progress of the reaction was monitored by thin-layer chromatography (petroleum ether: ethyl acetate = 1:5). After the reaction was completed, 30 mL of ice w...
Embodiment 3
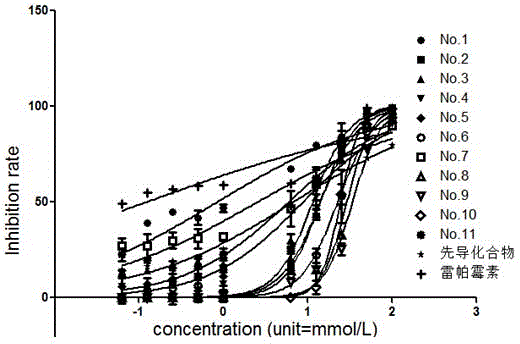
[0047] Example 3: CCK-8 method compound 4a, namely 3-(2,6-dichlorophenyl)-5-methyl-N-2-(6-methylsulfonylbenzothiazolyl)isoxazole-4-carboxamide Inhibition assay of Jurkat cells.
[0048] For Jurkat cells in good logarithmic phase, discard the supernatant after centrifugation, add 1ml of fresh 10% FBS RPMI-1640 complete culture solution, and gently blow the cells to avoid the generation of air bubbles, and prepare a single-cell suspension. For cell counting, dilute the cell suspension in proportion to make the cell density reach 6×105 cells / ml, and mix well. Plating: First, add 100 μL of PBS to the 36 wells around the experimental wells of the 96-well cell culture plate. Starting from well B2 of the culture plate, add 50 μL of cell suspension to the experimental wells in turn, and then add 200 mmol / L, 100 mmol / L, 50 mmol / L, 25 mmol / L, and 12.5 mmol to each experimental well in turn. / L, 2 mmol / L, 1 mmol / L, 500 mmol / L, 250 nmol / L, 125 nmol / L drug solutions containing N-contai...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com