Method for clean production of vanadium pentoxide
A vanadium pentoxide and clean production technology, applied in the field of clean production of vanadium pentoxide, can solve the problems of difficult comprehensive utilization, troughing, complicated procedures, etc., and achieve the effects of realizing full recycling, reducing production costs, and being easy to industrialize.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
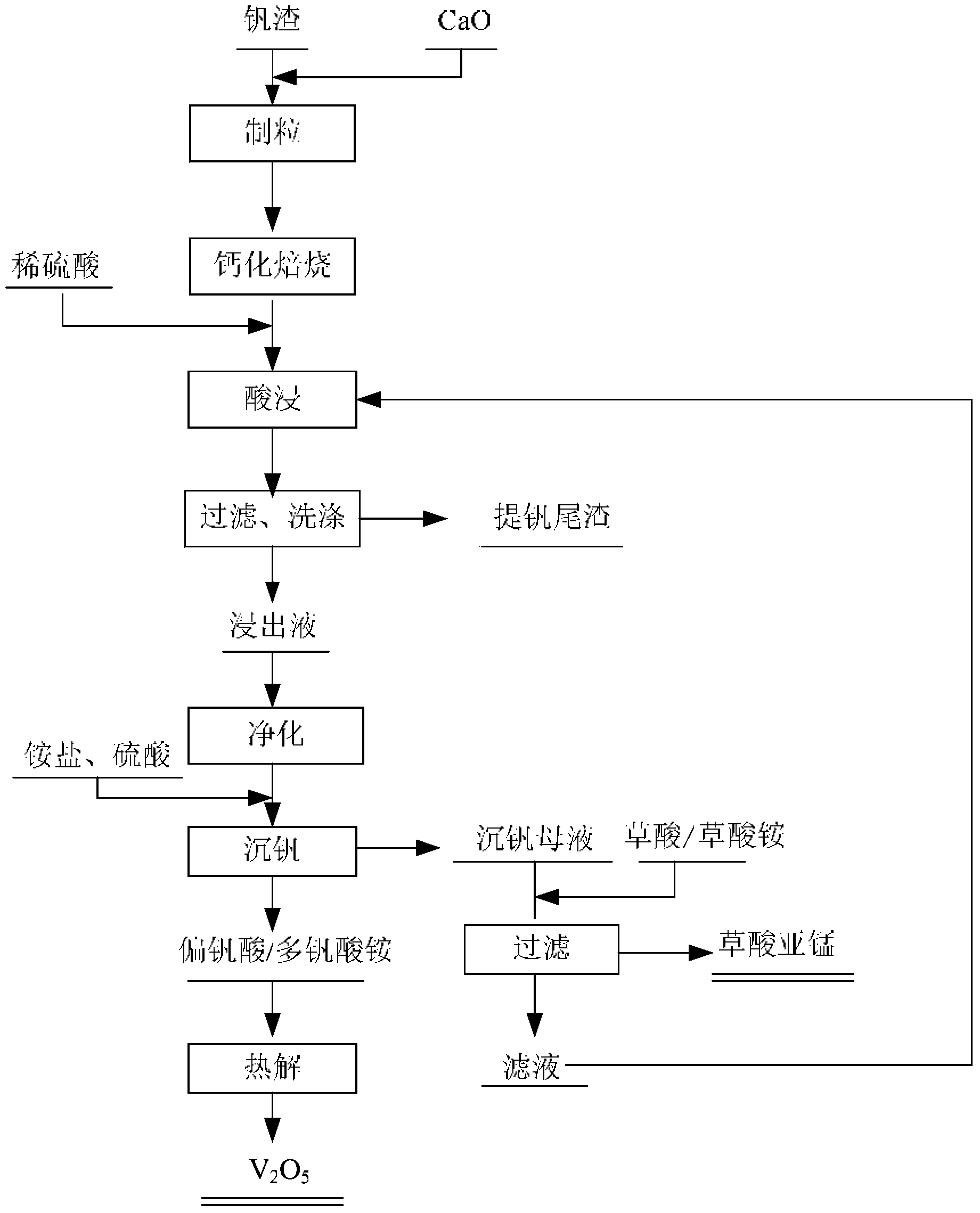
[0036] A method for cleanly producing vanadium pentoxide, comprising the steps of:
[0037] Step 1, raw material pretreatment: crush high-calcium vanadium slag to 200-260 mesh, add CaO to mix evenly, add water, and make raw meal balls with a particle size of 8-12mm; wherein, the mass ratio m 1 :m 2 is 0.42, mass ratio m 3 :m 4 is 0.02;
[0038] Step 2, roasting: heating the raw material balls to 800 °C at a heating rate of 4 °C / min, and keeping the temperature for 120 min to obtain roasted clinker balls;
[0039] Step 3, dilute acid leaching: the roasted clinker balls are crushed to 100-160 mesh, leached with dilute sulfuric acid solution, filtered to obtain vanadium leaching solution and vanadium extraction tailings, the vanadium extraction rate is 93.5%; vanadium extraction tailings are washed with water, and the washing water It can be used as the next dilute acid leaching roasting clinker leaching agent; among them, the concentration of dilute sulfuric acid solution is...
Embodiment 2
[0047]A method for cleanly producing vanadium pentoxide, comprising the steps of:
[0048] Step 1, raw material pretreatment: crush high-calcium vanadium slag to 200-400 mesh, add CaCO 3 Mix evenly and add water to make raw meal balls with a particle size of 9-12mm; among them, the mass ratio m 1 :m 2 is 1.0, the mass ratio m 3 :m 4 is 0.03;
[0049] Step 2, roasting: heating the raw material balls to 950 °C at a heating rate of 5 °C / min, and keeping the temperature for 15 minutes to obtain roasted clinker balls;
[0050] Step 3, dilute acid leaching: the roasted clinker balls are crushed to 160-200 mesh, leached with dilute sulfuric acid solution, filtered to obtain vanadium leaching solution and vanadium extraction tailings, the leaching rate is 93.3%; the vanadium extraction tailings are washed with water, and the washing water can be As the next dilute acid leaching roasting clinker leaching agent; wherein, the concentration of dilute sulfuric acid solution is 10%, th...
Embodiment 3
[0057] A method for cleanly producing vanadium pentoxide, comprising the steps of:
[0058] Step 1, raw material pretreatment: crush high-calcium vanadium slag to 200-300 mesh, add CaO to mix evenly, add water, and make raw meal balls with a particle size of 8-10mm; wherein, the mass ratio m 1 :m 2 is 0.5, mass ratio m 3 :m 4 is 0.04;
[0059] Step 2, roasting: heating the raw material balls to 850 °C at a heating rate of 1 °C / min, and keeping the temperature for 200 min to obtain roasted clinker balls;
[0060] Step 3, dilute acid leaching: the roasted clinker balls are crushed to 160-200 mesh, leached with dilute sulfuric acid solution, filtered to obtain vanadium leaching solution and vanadium extraction tailings, the leaching rate is 93.2%; the vanadium extraction tailings are washed with water, and the washing water can be As the next dilute acid leaching roasting clinker dissolution agent; wherein, the concentration of dilute sulfuric acid solution is 20%, the liquid...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com