Indomethacin cataplasm and composition thereof
A technology of indomethacin and indomethacin, applied in the direction of drug combinations, anti-inflammatory agents, non-central analgesics, etc., can solve the problems of poor transdermal absorption performance, low solubility, easy decomposition, etc., and increase transdermal absorption Performance, increased transdermal absorption, long-term moisturizing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
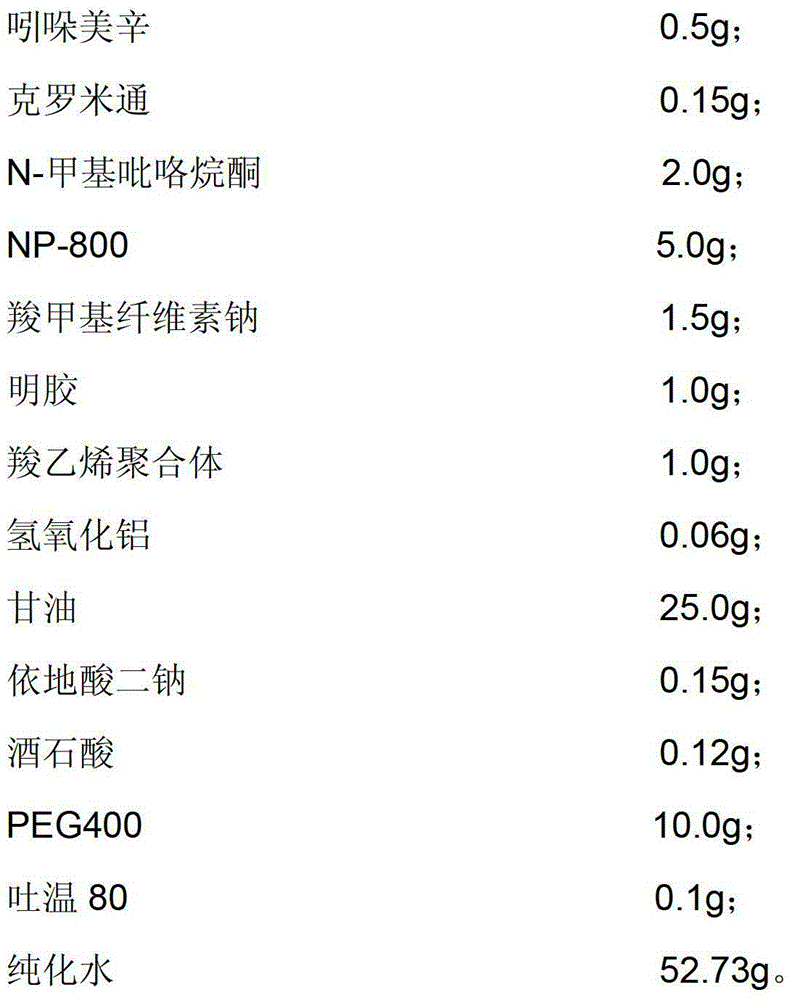
[0049] The indomethacin cataplasm provided in this embodiment is composed of a backing, a paste and an anti-adhesive film, and the composition of the paste includes:
[0050]
[0051] Dissolve 2.0g of sodium carboxymethylcellulose in glycerin and partially purified water, then add 1.0g of polyoxyethylene monolaurate and 0.1g of Tween 80, heat to 40°C, and stir well to obtain component A;
[0052] Dissolve 1g of gelatin in partially purified water, heat to 60°C after swelling is complete, stir evenly until translucent colloid, and obtain component B;
[0053] Dissolve 5.0g of partially neutralized sodium polyacrylate, 0.06g of aluminum hydroxide and 0.15g of disodium edetate in glycerin, stir and mix evenly to obtain component C;
[0054] Add 0.12 g of tartaric acid into purified water, then add indomethacin solution pre-dissolved in crotamiton and N-methylpyrrolidone, mix and stir evenly to obtain component D;
[0055] Take the remaining purified water in the prefabricated...
Embodiment 2
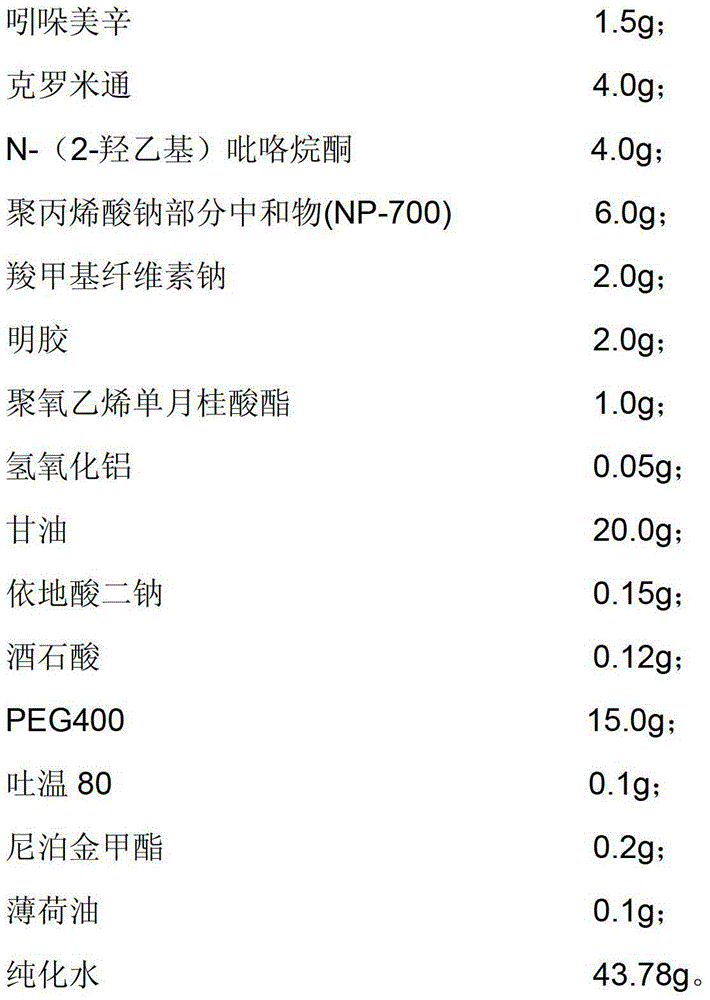
[0057] The indomethacin cataplasm provided in this embodiment is composed of a backing, a paste and an anti-adhesive film, and the composition of the paste includes:
[0058]
[0059] Dissolve 2.0g of sodium carboxymethylcellulose in glycerin and partially purified water, then add 1.0g of polyoxyethylene monolaurate and 0.1g of Tween 80, heat to 40°C, and stir well to obtain component A;
[0060] Dissolve 2.0g of gelatin in partially purified water, heat to 60°C after swelling is complete, and stir evenly until translucent colloid is obtained to obtain component B;
[0061] Dissolve 6.0g of partially neutralized sodium polyacrylate, 0.05g of aluminum hydroxide and 0.15g of disodium edetate in glycerin, stir and mix evenly to obtain component C;
[0062] Add 0.12 g of tartaric acid into purified water, then add indomethacin solution pre-dissolved in crotamiton and N-methylpyrrolidone, mix and stir evenly to obtain component D;
[0063] Take the remaining purified water in a...
Embodiment 3
[0065] The indomethacin cataplasm provided in this embodiment is composed of a backing, a paste and an anti-adhesive film, and the composition of the paste includes:
[0066]
[0067]
[0068] Dissolve 2.0g sodium carboxymethylcellulose in glycerin and partially purified water, then add 1.0g carboxyvinyl polymer, 0.3g polyoxyethylene hydrogenated castor oil and 0.1g Tween 80, heat to 40°C, stir well, Get A component.
[0069] Dissolve 1.0g of gelatin in partially purified water, heat to 60°C after swelling is complete, and stir evenly until translucent colloid is obtained to obtain component B;
[0070] Dissolve 6.0g of partially neutralized sodium polyacrylate, 0.05g of aluminum hydroxide and 0.15g of disodium edetate in glycerin, stir and mix evenly to obtain component C;
[0071] Add 0.12 g of tartaric acid into purified water, then add indomethacin solution pre-dissolved in crotamiton and N-methylpyrrolidone, mix and stir evenly to obtain component D;
[0072] Take...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Skin penetration rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com