Production method of cyclopentyl methyl ether
A technology of cyclopentyl methyl ether and its production method, which is applied in chemical instruments and methods, preparation of ether, purification/separation of hydrocarbons, etc., can solve the problems of catalyst activity decline, low volume space velocity, resin catalyst loss of activity, etc., and achieve The effect of improving activity stability, prolonging life and service life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~10
[0025] In the following examples, the cyclopentene is referred to as CPE for short, and the cyclopentyl methyl ether is referred to as CPME for short.
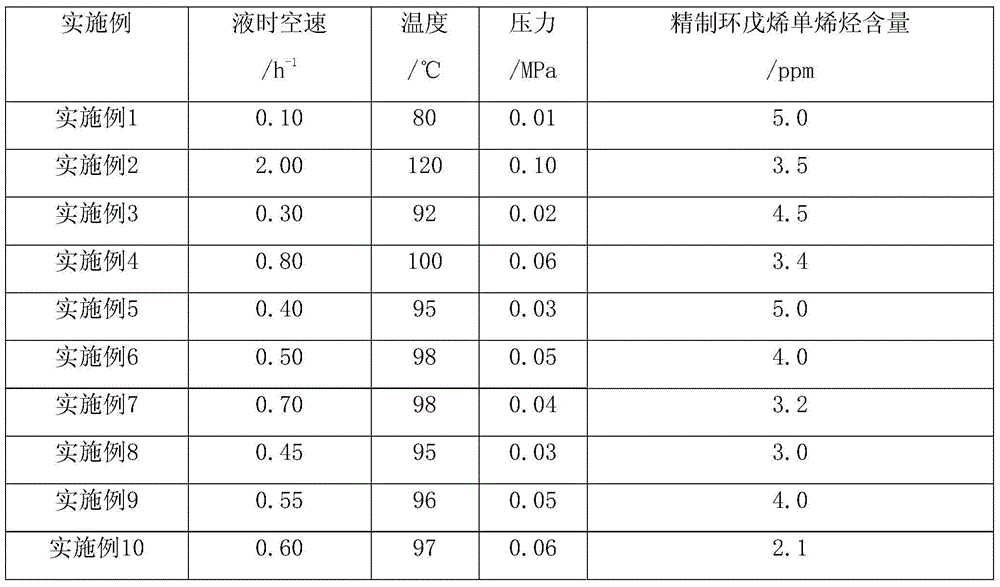
[0026] The refining reactor of cyclopentene is a stainless steel tubular reactor of φ25mm×1000mm. The reaction tube is filled with 100ml granular clay with a particle size of 20-60 mesh and acidity ≤2.5KOHmg / g. The packing density of the granular clay fixed bed is 0.65~0.70g / ml. The bottom of the reactor is filled with inert ceramic balls. After the cyclopentene raw material is preheated, the pump is used to send the refined cyclopentene material from the top to the refining reactor at a set rate, and the refined cyclopentene material flowing out of the outlet is used as the etherification reaction raw material after it is cooled to room temperature. The composition of cyclopentene to be refined is shown in Table 1, and the conditions and results of cyclopentene refining are shown in Table 2.
Embodiment 11~20
[0028] The purified cyclopentene obtained in Examples 1-10 were subjected to etherification reactions in Examples 11-20 respectively.
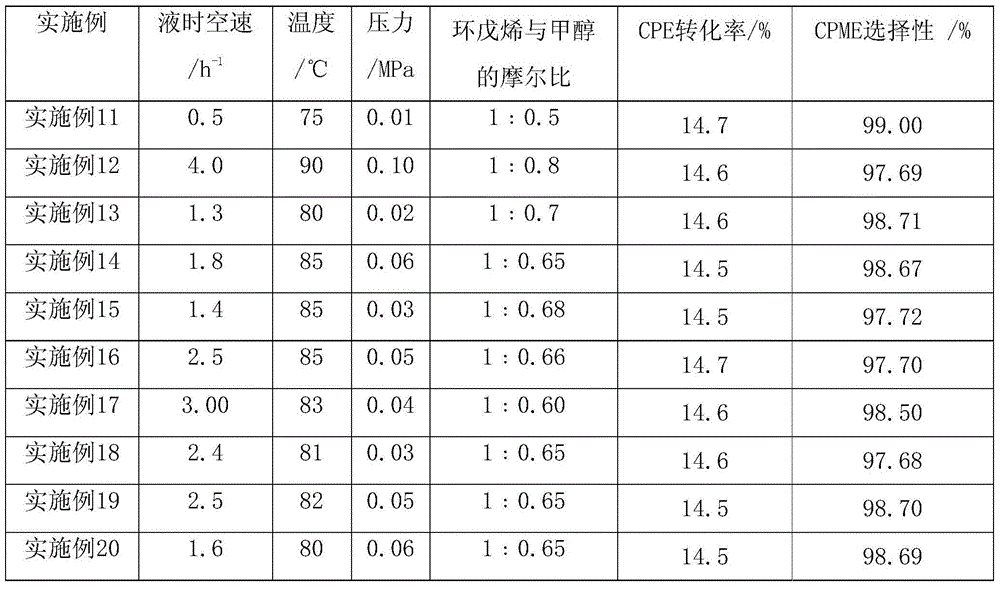
[0029]The etherification reactor is a stainless steel tubular reactor with a diameter of φ25mm×1000mm. The reaction tube is filled with 100ml of spherical sulfonic acid-based cation exchange resin catalyst with a particle size of 0.40-1.25mm to form a fixed-bed catalyst bed. The mass exchange of the resin The capacity is 3~5.5mmol / g. The outside of the reactor is equipped with a temperature-controlled jacket of circulating hot water, and platinum resistors for temperature measurement are respectively installed on the upper, middle and lower parts of the catalyst bed. The amount of reaction feed is controlled by a feed pump, and the system pressure is regulated by a back pressure valve. The conversion and selectivity of the etherification reaction with a running time of 300 hours are shown in Table 3.
Embodiment 21
[0031] During the etherification reaction, the volume liquid hourly space velocity of the material is 1.5hr -1 , the system pressure is controlled at 0.04MPa, the feed temperature is 80°C, and the molar ratio of cyclopentene to methanol is 1:0.6. The relationship between the conversion rate of the etherification reaction and the running time is shown in Table 4.
[0032] Table 1.
[0033] components
Content (wt)%
1-pentene
0.0021
2-Methyl-1-butene
0.0065
2-Transpentene
0.1400
2-cis-pentene
0.1000
2-Methyl-2-butene
0.0222
98.2690
1.4600
[0034] Table 2.
[0035]
[0036] table 3.
[0037]
[0038] Table 4
[0039]
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| Bronsted acidity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com