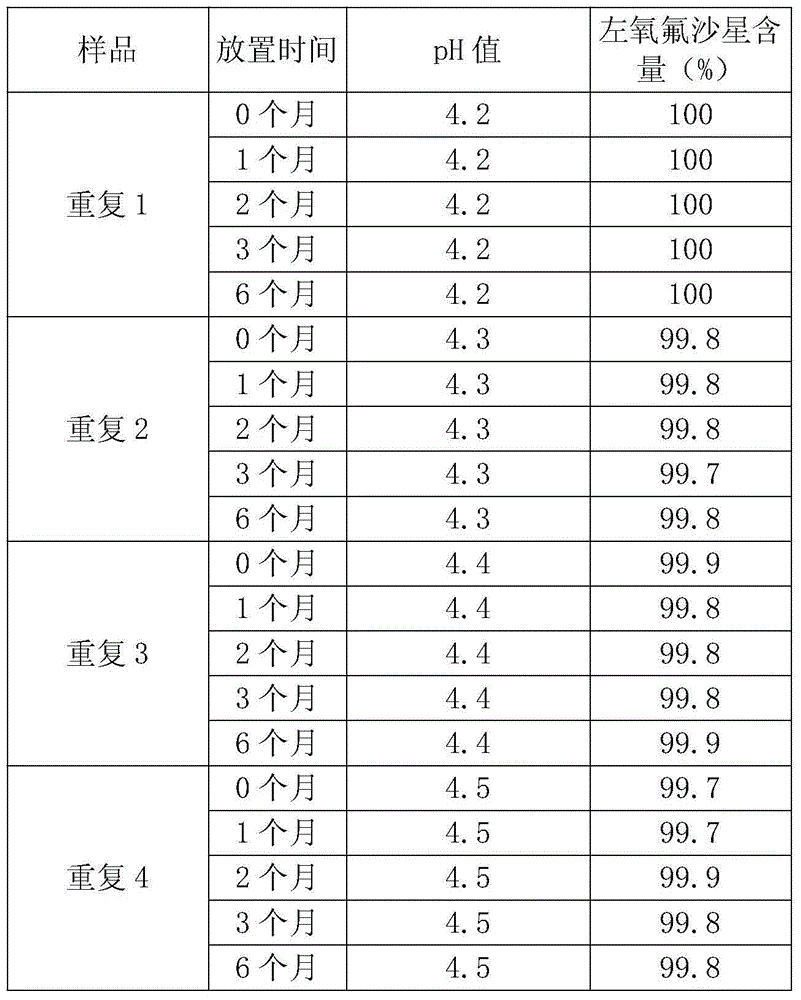
Preparation method of veterinary compound levofloxacin injection
The technology of compound levofloxacin and injection is applied in the field of preparation of compound levofloxacin injection for veterinary use, and can solve the problems of easy crystallization failure, occurrence of sepsis, unstable quality and the like, and achieve the effects of ensuring appetite, maintaining vitality and increasing appetite.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Prepare veterinary compound levofloxacin injection according to the following steps:
[0017] 1) Dissolve 45g of levofloxacin hydrochloride with water for injection, add 20ml of propylene glycol, stir and dissolve, and set aside;
[0018] 2) Add 45g of aminopyrine, 35g of nicotinamide, 60ml of propylene glycol, 80ml of N,N-dimethylformamide, and 0.8g of disodium edetate into water for injection, stir to dissolve, and set aside;
[0019] 3) Stir and dissolve 0.8 g of racecolin with water for injection, and set aside;
[0020] 4) Mix the liquids prepared in steps 1, 2, and 3 together, adjust the pH value to 4.0-4.5 with glacial acetic acid, add water for injection to make up to 1000ml, filter aseptically, and potting.
Embodiment 2
[0022] Prepare veterinary compound levofloxacin injection according to the following steps:
[0023] 1) Dissolve 50g of levofloxacin hydrochloride with water for injection, add 30ml of propylene glycol and stir to dissolve, set aside;
[0024] 2) Add 50g of aminopyrine, 40g of nicotinamide, 70ml of propylene glycol, 100ml of N,N-dimethylformamide, and 1.0g of disodium edetate into water for injection, stir to dissolve, and set aside;
[0025] 3) Stir and dissolve 1.0 g of racecolin with water for injection, and set aside;
[0026] 4) Mix the liquids prepared in steps 1, 2, and 3 together, adjust the pH value to 4.0-4.5 with glacial acetic acid, add water for injection to make up to 1000ml, filter aseptically, and potting.
Embodiment 3
[0028] Prepare veterinary compound levofloxacin injection according to the following steps:
[0029] 1) Dissolve 55g of levofloxacin hydrochloride with water for injection, add 30ml of propylene glycol and stir to dissolve, set aside;
[0030] 2) Add 55g of aminopyrine, 45g of nicotinamide, 90ml of propylene glycol, 120ml of N,N-dimethylformamide, and 1.2g of disodium edetate into water for injection, stir to dissolve, and set aside;
[0031] 3) Stir and dissolve 1.2 g of racecolin with water for injection, and set aside;
[0032] 4) Mix the liquids prepared in steps 1, 2, and 3 together, adjust the pH value to 4.5 with glacial acetic acid, add water for injection to make the volume up to 1000ml, filter aseptically, and seal it.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com