Novel preparation method for vitamin D class drug
A technology for compound and silyl ether protection, applied in the field of vitamin D drug synthesis, can solve the problems of cumbersome separation and purification of intermediates and products, inability to realize industrialization, low yield, etc., and achieves good reaction orientation selectivity and operation process The effect of short time and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
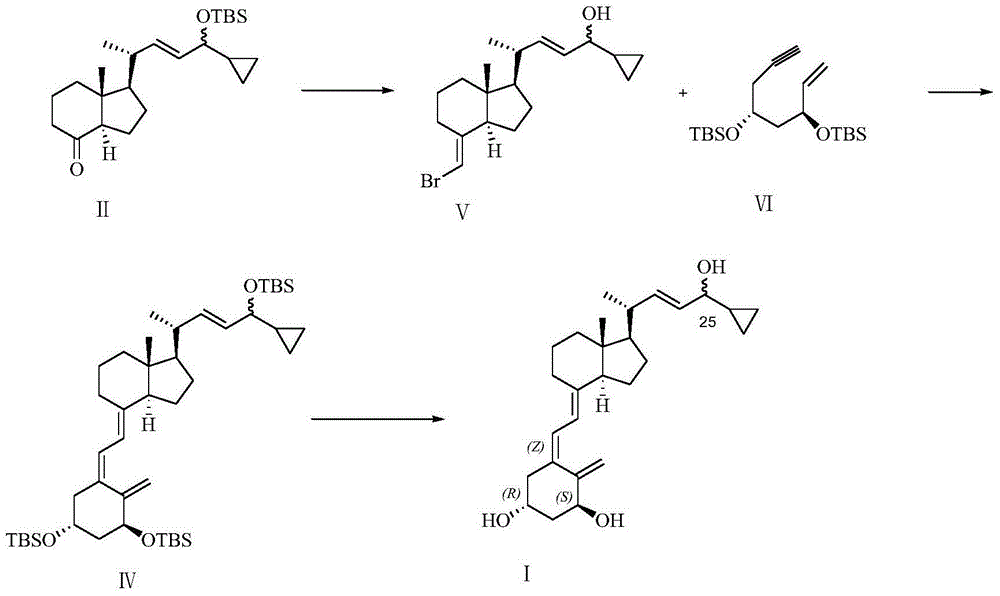
[0041] Part I Preparation of silyl ether protected calcipotriol
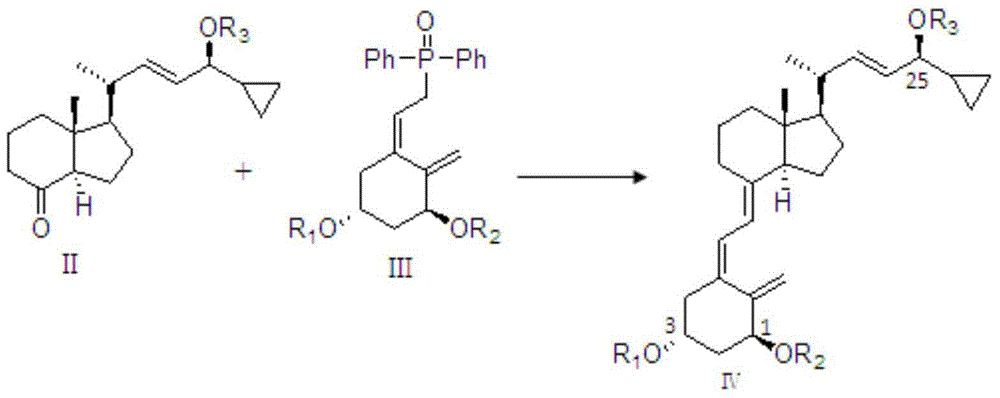
[0042] The preparation of silicon ether-protected calcipotriol is as follows: the compound shown in formula II and the compound shown in formula III are subjected to double bond coupling reaction under basic conditions to obtain the compound shown in formula IV. In the formula, R 1 , R 2 , R 3 The silicon ether protecting group is any one or more of dimethyl tert-butylsilane, triisopropylsilane, tert-butyldiisopropylsilane, triethylsilane and trimethylsilane. The above compounds are dissolved in organic solvents. The organic solvent includes any one or more of tetrahydrofuran, methyltetrahydrofuran, diethyl ether and toluene. In order to ensure that the reaction proceeds in an alkaline environment, alkaline reagents such as potassium dimethylsilylamide and potassium trimethylsilylamide can be added.
[0043]
Embodiment 1
[0044] Example 1 Preparation of silicon ether protected calcipotriol (compound of formula IV)
[0045] Under the protection of nitrogen, add 5ml of tetrahydrofuran and 5g of the compound of formula III into a 25ml dry single-necked bottle, cool to -80~-60℃, add 8ml of bis(trimethylsilyl) sodium amide solution dropwise, and control the temperature at -80~- After 0.5 hour at 60°C, slowly drop in a solution of 3g of the compound of formula II dissolved in 3ml of tetrahydrofuran, control the temperature at -80 to -60°C for 1 to 3 hours, and monitor by TLC (the developer is n-hexane: ethyl acetate = 30:1), After the reaction was completed, the temperature was raised to -5-5°C, and saturated ammonium chloride aqueous solution was added to the system to quench the reaction, and then extracted three times with ethyl acetate, the organic layer was washed twice with water, and dried over anhydrous sodium sulfate. After filtration and concentration, the resulting residue was added to (et...
Embodiment 2
[0046] Example 2 Preparation of silicon ether protected calcipotriol (compound of formula IV)
[0047] Under the protection of nitrogen, add 10ml of tetrahydrofuran and 2g of the compound of formula III to a 25ml dry single-necked bottle, cool to -80~0℃, add dropwise 4ml of bis(trimethylsilyl)potassium amide solution, and control the temperature at -80~-60 After 0.5 hours at ℃, slowly drop into the solution of 1.3g of the compound of formula II dissolved in 5ml of tetrahydrofuran, control the temperature at -80~-60℃ for 1~3 hours, and monitor the reaction by TLC (developing agent is n-hexane:ethyl acetate=30:1) After completion, the temperature was raised to -5-5°C, and saturated ammonium chloride aqueous solution was added to the system to quench the reaction, and then extracted three times with ethyl acetate, the organic layer was washed twice with water, and dried over anhydrous sodium sulfate. The residue obtained after concentration was added to (ethyl acetate:n-hexane=1:...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com