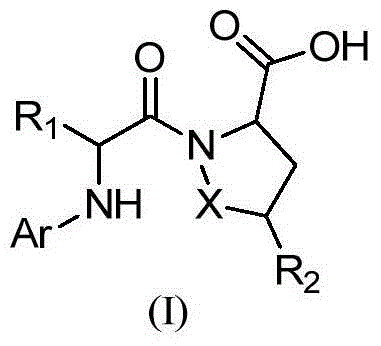
Anticoagulant compound, preparation method and application of anticoagulant compound and drug composition containing anticoagulant compound
A compound and drug technology, applied in the field of pharmaceutical compositions containing the compound and anticoagulant compounds, can solve problems such as thrombus, and achieve the effects of high safety, good application prospects and novel structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
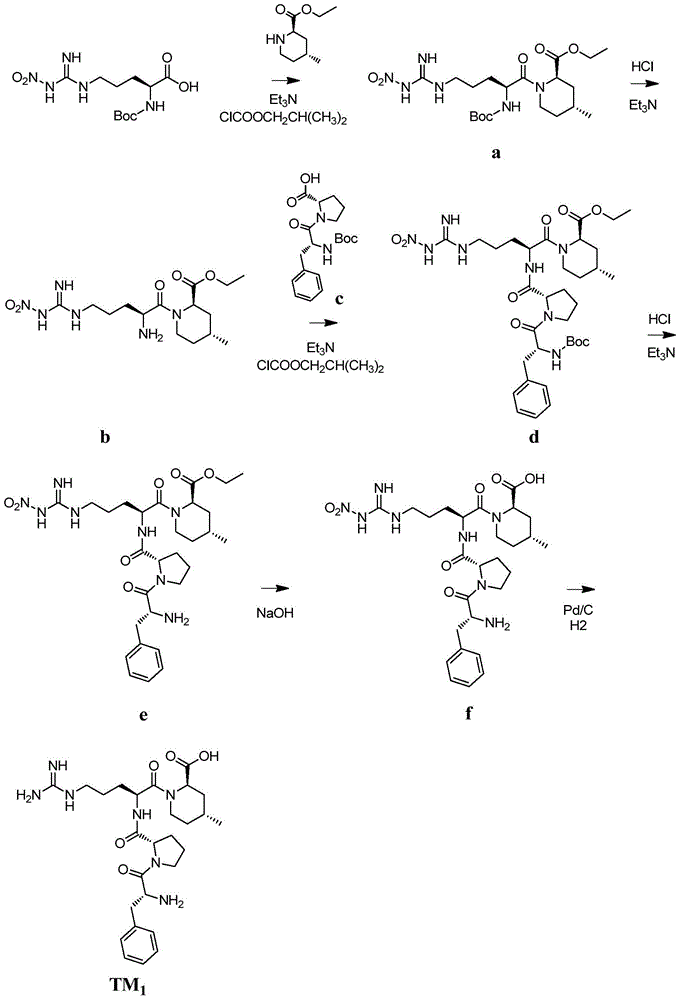
[0071] Example 1TM 1 preparation of
[0072]
[0073] Such as figure 1 As shown, the synthesis steps are as follows:
[0074] 1) Add 350mL dry tetrahydrofuran to the reaction flask, add N G -Nitro-N 2 -Boc-L-arginine (31.9g, 0.1mol, purchased from Jill Biochemical (Shanghai) Co., Ltd.), cooling the reaction solution to -20°C, adding triethylamine (10.1g, 0.1mol) and Isobutyl chloroformate (13.6 g, 0.1 mol). After 30 minutes, (2R, 4R)-4-methyl-2-piperidinecarboxylic acid ethyl ester (20.5g, 0.12mol) was added to the reaction solution, and the temperature of the reaction solution was lowered after stirring for 30 minutes at -20°C. Return to room temperature. After the reaction solution was concentrated under reduced pressure, 350 mL of ethyl acetate was added, and after dissolution, the organic layer was washed with 200 mL of water, 100 mL of 5% sodium bicarbonate solution, 100 mL of 10% citric acid solution and 200 mL of water. Ethyl acetate was dried over anhydrous s...
Embodiment 2
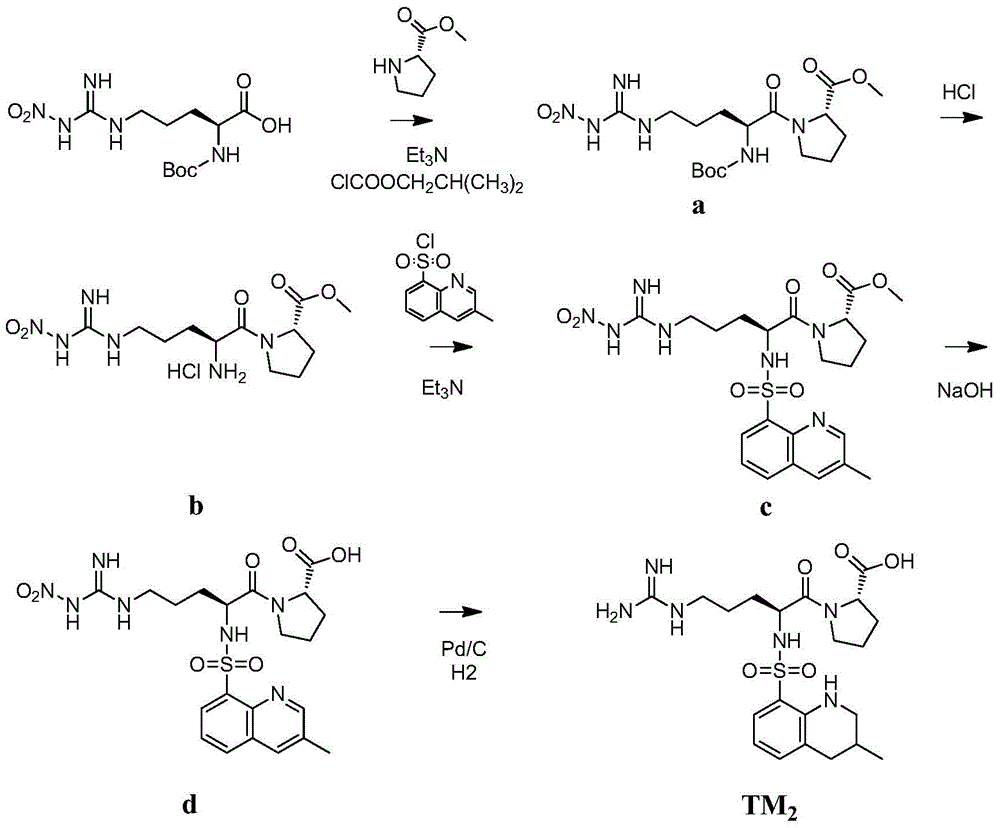
[0084] Example 2TM 2 preparation of
[0085]
[0086] Such as figure 2 As shown, the synthesis steps are as follows:
[0087] 1) Dissolve L-proline methyl ester hydrochloride (44.1g, 0.267mol) in dichloromethane, add 37.10ml triethylamine under stirring condition, white flocs are formed, filter the precipitate, and the filtrate is reduced Concentrate under reduced pressure to obtain white solid L-proline methyl ester, which is directly used for the next reaction without further treatment.
[0088] Add 300 mL of dry tetrahydrofuran to the reaction flask, add N G -Nitro-N 2 -Boc-L-arginine (28.4g, 0.089mol), cooled the reaction solution to -20°C, and added triethylamine (9.0g, 0.089mol) and isobutyl chloroformate (12.1g, 0.089 mol). After 30 minutes, L-proline methyl ester was added to the reaction solution, stirred at -20° C. for 30 minutes, and then the temperature of the reaction solution was returned to room temperature. After the reaction solution was concentrate...
Embodiment 3
[0094] Example 3TM 1 injection
[0095] Specifications and composition:
[0096] The capacity of each injection is 2.5mL, and each 1mL injection contains 100mg TM 1 , 300mg D-sorbitol and 400mg absolute ethanol.
[0097] Preparation:
[0098] Add 200g water for injection into a 1000mL beaker, add 300g D-sorbitol to it, stir to dissolve (heat if necessary); then add 400g absolute ethanol, stir and add 100g TM 1 , and stir until completely dissolved. Sterilized by filtration, filled to get 2.5mL / bottle, each 1mL contains 100mg TM 1 injection.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com