Electrolytic corrosion agent capable of displaying nickel-based corrosion-resistant alloy metallographic structure and corrosion method thereof
A technology of corrosion-resistant alloy and metallographic structure, applied in the field of metallographic electrolytic corrosion, can solve the problems of personal safety and health injury of experimenters, changes in concentration ratio, environmental pollution, etc., and achieve optimized electrolysis time, stable properties, and corrosion effects Good results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
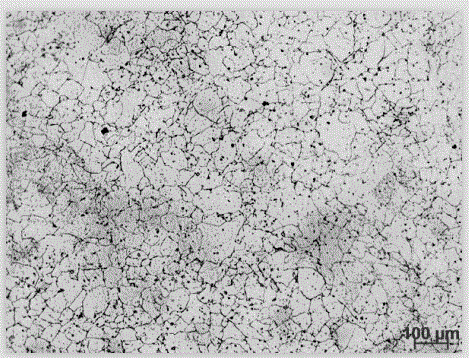
[0022] (1) Preparation of electrolytic corrosion agent: Weigh 40g of oxalic acid, 180g of glycerin, and 500ml of deionized water. At room temperature, pour oxalic acid, glycerin, and deionized water into a glass container in turn, and stir with a glass rod until the oxalic acid is completely dissolved to obtain the electrolytic corrosion agent. corrosive;
[0023] (2) Polish the nickel-based corrosion-resistant alloy Inconel625 to be corroded with 400#, 600#, 800#, 1000# water-grinding sandpaper, and then mechanically polish it with diamond polishing agent;
[0024] (3) Pour the electrolytic corrosion agent prepared in step (1) into the electrolytic corrosion instrument. The cathode material of the electrolytic corrosion instrument is 304 stainless steel, and the polished nickel-based corrosion-resistant alloy Inconel625 is used as the anode. The electrolytic corrosion DC voltage is 20V and the current is 6.0A, the electrolytic corrosion temperature is room temperature, and th...
Embodiment 2
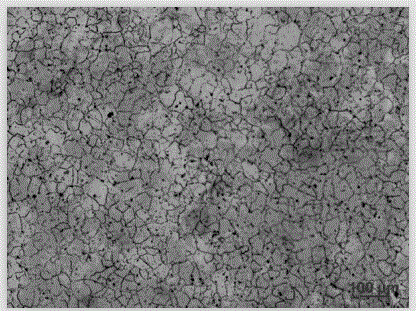
[0028] (1) Preparation of electrolytic etchant: Weigh 50g of oxalic acid, 150g of glycerin, and 450ml of deionized water. At room temperature, pour oxalic acid, glycerin and deionized water into a glass container in turn, and stir with a glass rod until the oxalic acid is completely dissolved to prepare an electrolytic etchant. .
[0029] (2) Polish the nickel-based corrosion-resistant alloy Inconel625 to be corroded with 400#, 600#, 800#, 1000# water-grinding sandpaper, and then mechanically polish it with diamond polishing agent.
[0030] Pour the electrolytic corrosion agent prepared in step (1) into the electrolytic corrosion instrument. The cathode material of the electrolytic corrosion instrument is 304 stainless steel, and the polished nickel-based corrosion-resistant alloy is used as the anode. The electrolytic corrosion DC voltage is 18V and the current is 5.5A. The corrosion temperature is room temperature, and the electrolytic corrosion time is 20s. After the c...
Embodiment 3
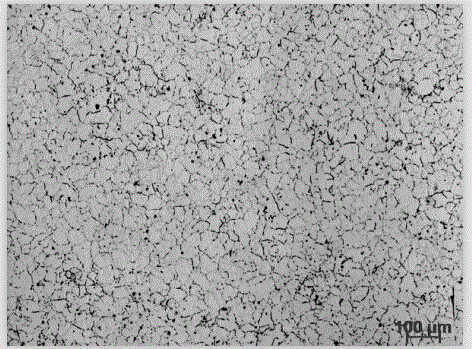
[0033] (1) Preparation of electrolytic etchant: Weigh 60g of oxalic acid, 120g of glycerin, and 400ml of deionized water. At room temperature, pour oxalic acid, glycerin and deionized water into a glass container in turn, and stir with a glass rod until the oxalic acid is completely dissolved to prepare an electrolytic etchant. .
[0034] (2) Polish the nickel-based corrosion-resistant alloy Inconel800 to be corroded with 400#, 600#, 800#, 1000# water-grinding sandpaper, and then mechanically polish it with diamond polishing agent.
[0035] (3) Pour the electrolytic corrosion agent prepared in step (1) into the electrolytic corrosion instrument. The cathode material of the electrolytic corrosion instrument is 304 stainless steel, and the polished nickel-based corrosion-resistant alloy is used as the anode. The electrolytic corrosion DC voltage is 15V and the current is 5.0 A, the electrolytic corrosion temperature is room temperature, and the electrolytic corrosion time is...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com