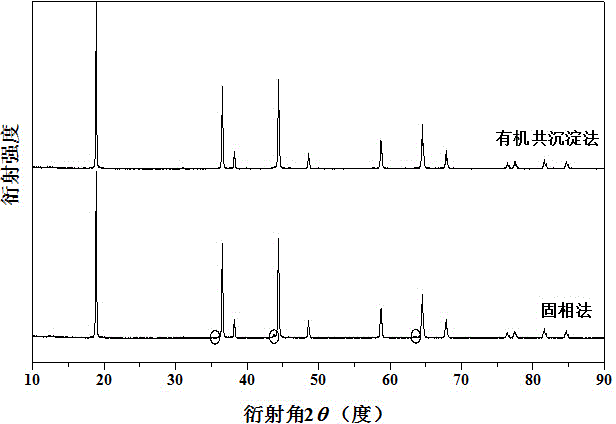
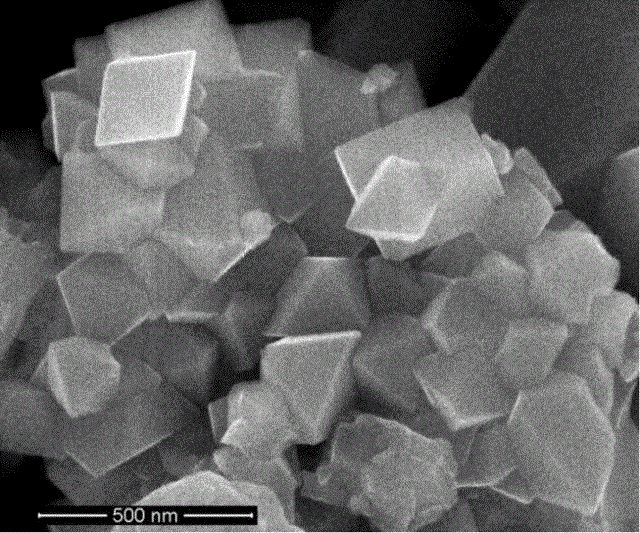
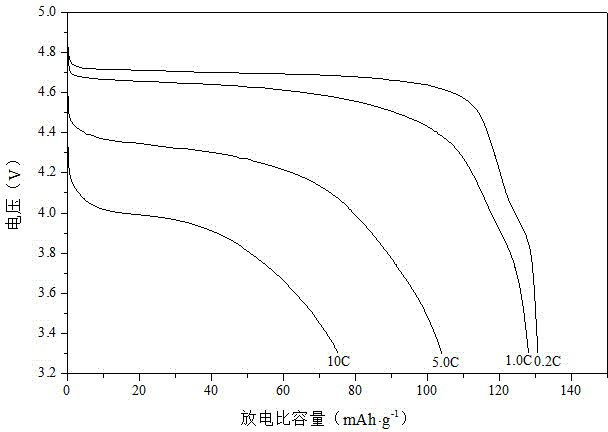
Preparation method of nickel-manganese spinel high-voltage positive material of lithium secondary battery
A lithium secondary battery and positive electrode material technology, applied in battery electrodes, circuits, electrical components, etc., can solve the problems of unbalanced element loss, difficult control of product stoichiometric ratio, uncertainty, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Add 0.02mol of lithium acetate, 0.01mol of nickel acetate, and 0.03mol of manganese acetate into deionized water, dissolve, stir and mix uniformly to obtain mixed solution A; weigh 0.08mol of organic precipitant 8-hydroxyquinoline, and dissolve at 60°C In ethanol, solution B was obtained. Under continuous magnetic stirring, solution A was slowly added dropwise to solution B, and stirring was continued for 2 hours after the addition was completed, during which precipitation was continuously formed. The precipitated dispersion was evaporated in a water bath at 80°C to remove the solvent, and then dried in a blast oven at 110°C for 10 hours to obtain a precipitate. The obtained precipitate was pre-calcined at 300° C. for 2 h to obtain a precursor. After the precursor was ground, it was pressed into pellets under a pressure of 10 MPa. In an air atmosphere, the temperature was raised to 800 °C at a rate of 10 °C / min and kept for 10 h, and then cooled to room temperature at ...
Embodiment 2
[0037] Add 0.03mol lithium oxalate, 0.015mol nickel oxalate, and 0.045mol manganese oxalate into deionized water respectively, dissolve, stir and mix uniformly to obtain a mixed solution A; weigh 0.10mol organic precipitant dinitroaniline, and dissolve it in In cyclohexane, solution B was obtained. Under continuous magnetic stirring, solution B was slowly added dropwise to solution A, and stirring was continued for 3 hours after the addition was completed, during which precipitation was continuously formed. The precipitated dispersion was evaporated in a water bath at 80°C to remove the solvent, and then dried under vacuum at 120°C overnight to obtain a precipitate. The obtained precipitate was pre-calcined at 400° C. for 5 h to obtain a precursor. After the precursor was ground, it was pressed into pellets under a pressure of 8 MPa. Under an oxygen atmosphere, the temperature was raised to 750 °C at a rate of 5 °C / min and kept for 20 h, and then cooled to room temperature at...
Embodiment 3
[0039] Add 0.02mol of lithium hydroxide, 0.01mol of nickel chloride, and 0.03mol of manganese chloride into deionized water respectively, dissolve, stir and mix uniformly to obtain a mixed solution A; weigh 0.06mol of organic precipitant dimethylglyoxime, and place Dissolve in isopropanol to obtain solution B. Under continuous magnetic stirring, slowly add solution A and solution B into the beaker drop by drop at the same time. During this process, precipitates are continuously formed, and stirring is continued for 5 hours after the dropwise addition is completed. The precipitate dispersion system was evaporated in a water bath at 70°C to remove the solvent, and then dried in a blast drying oven at 120°C for 12 hours to obtain a precipitate. The obtained precipitate was pre-calcined at 470° C. for 3 h to obtain a precursor. After the precursor is ground, it is pressed into pellets under a pressure of 12MPa, and the temperature is raised to 850°C at a rate of 15°C / min under a ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com