Composite all-solid-state polymer electrolyte and preparation method thereof
An all-solid polymer and electrolyte technology, which is applied in the manufacture of electrolyte batteries, non-aqueous electrolyte batteries, circuits, etc., can solve the problems of unstable electrochemical properties of electrolytes, achieve improved interface compatibility, improve mechanical properties, and improve electrochemical performance. stable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~4
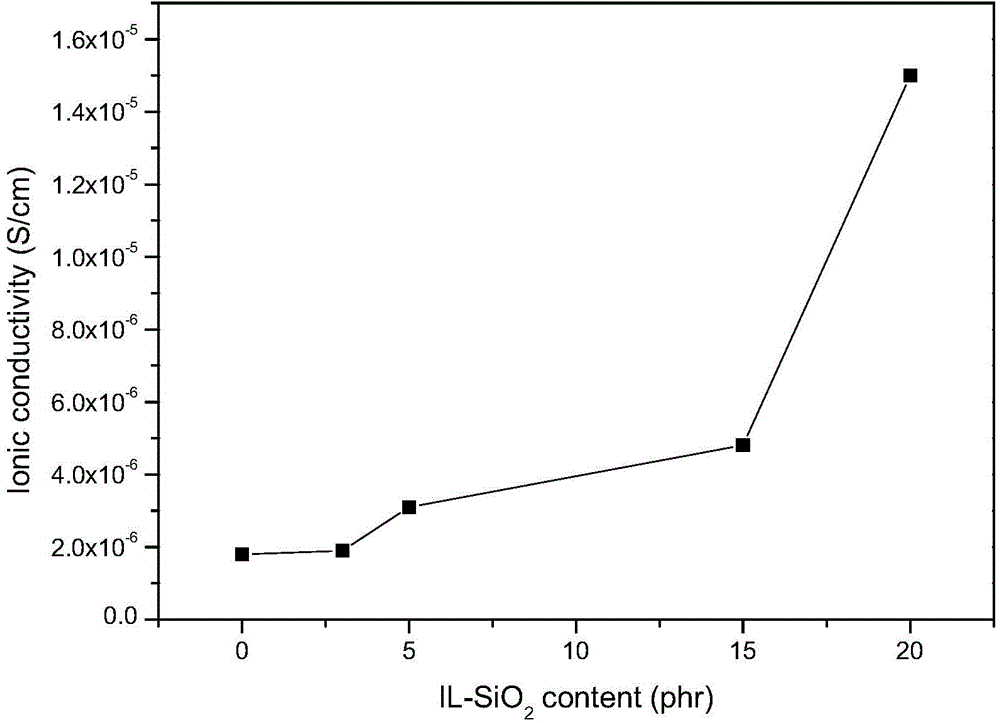
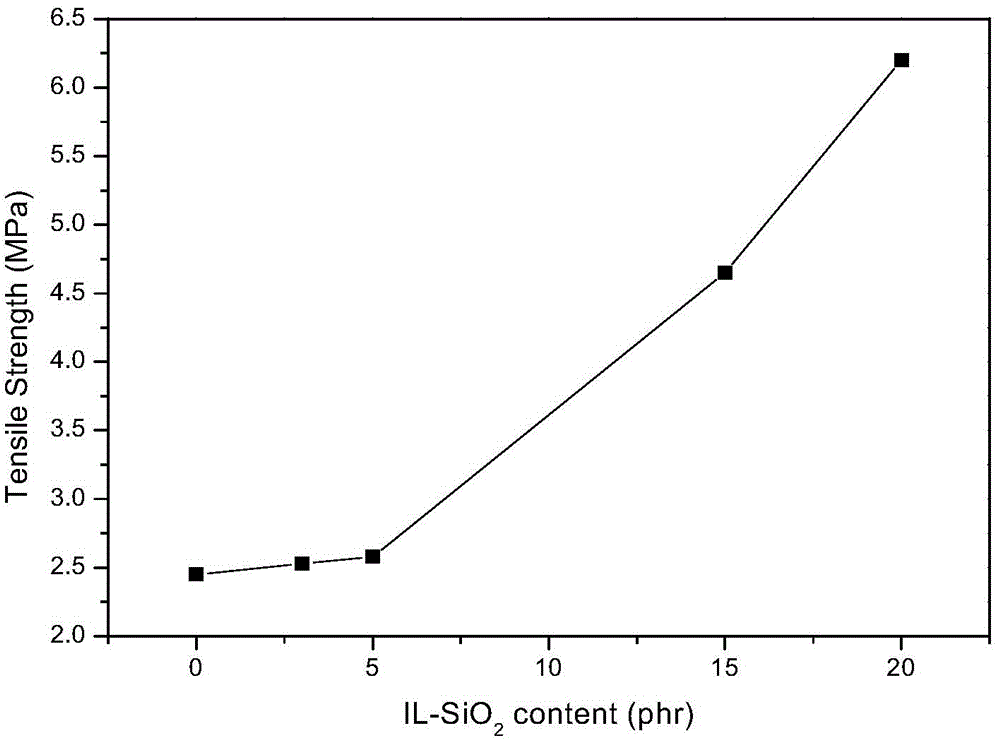
[0046] (1)IL-SiO2 2 preparation of
[0047] Add a certain proportion of polyoxyethylene-polyoxypropylene-polyoxyethylene block copolymer (P123), 12mol / L HCl aqueous solution, and deionized water into the flask, stir at 40°C until P123 is completely dissolved, and orthosilicic acid Add ethyl ester to the flask, continue stirring for 24 hours, raise the temperature to 90°C, stop stirring, crystallize for 24 hours, wash with deionized water and ethanol after separation by suction filtration, extract at 130°C for 24 hours, and extract the powder Dry in a vacuum oven at 80°C for 4 hours to obtain mesoporous SiO 2 .
[0048] Mesoporous SiO 2Disperse in dewatered DMF solvent and sonicate for 1h. Dissolve the quantitative IL in the dewatered dimethylformamide (DMF) solvent, and add dropwise 4-dimethylaminopyridine (DMAP) and dicyclohexyl in the ice-water bath and stirring condition, respectively. Carbodiimide (DCC), after 1h dropwise addition of mesoporous SiO 2 Dispersions. Af...
Embodiment 5
[0070] A composite all-solid polymer electrolyte, which is prepared by using the raw materials of the following components and contents;
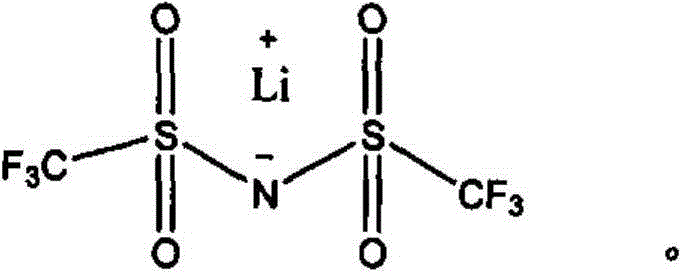
[0071] Carboxylated nitrile rubber 1kg; vulcanizing agent 0.02kg; lithium salt 0.2kg; IL-SiO 2 0.01kg. Among them, the Mooney viscosity ML of carboxylated nitrile rubber 1+4 It is 40 at 100°C, wherein the content of acrylonitrile is 27wt%, and the content of carboxyl group is 5wt%. The vulcanizing agent is dicumyl peroxide. The lithium salt is lithium bistrifluoroformimide, and its structural formula is as follows:
[0072]
[0073] IL-SiO 2 It is a complex of 1-carboxymethyl-3-methylimidazole bistrifluoromethanesulfonimide salt (ionic liquid, abbreviated as IL) and mesoporous silica. The molecular structure of IL is as follows:
[0074]
[0075] Mesoporous SiO 2 The pore size is 4nm, and it is prepared by the following method: add polyoxyethylene-polyoxypropylene-polyoxyethylene block copolymer, 12mol / L HCl aqueous solution, an...
Embodiment 6
[0086] A composite all-solid polymer electrolyte, which is prepared by using the following components and contents of raw materials:
[0087] Carboxylated nitrile rubber 1kg; vulcanizing agent 0.03kg; lithium salt 0.6kg; IL-SiO 2 0.5kg. Among them, the Mooney viscosity ML of carboxylated nitrile rubber i+4 It is 50 at 100°C, wherein the acrylonitrile content is 29 wt%, and the carboxyl content is 7 wt%. The vulcanizing agent is dicumyl peroxide. The lithium salt is lithium bistrifluoroformimide, and its structural formula is as follows:
[0088]
[0089] IL-SiO 2 It is a complex of 1-carboxymethyl-3-methylimidazole bistrifluoromethanesulfonimide salt (ionic liquid, abbreviated as IL) and mesoporous silica. The molecular structure of IL is as follows:
[0090]
[0091] Mesoporous SiO 2The pore size is 8nm, and it is prepared by the following method: add polyoxyethylene-polyoxypropylene-polyoxyethylene block copolymer, 12mol / L HCl aqueous solution, and deionized wate...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Aperture | aaaaa | aaaaa |
| Ionic conductivity at room temperature | aaaaa | aaaaa |
| Aperture | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com