Efficient heavy-metal-ion absorbent material and preparation method thereof
A technology of heavy metal ions and adsorption materials, applied in chemical instruments and methods, adsorption water/sewage treatment, other chemical processes, etc., can solve the lack of designability and controllability of the adsorption process, and the difficulty in realizing heavy metal recovery and reuse of adsorbents , It is difficult to adsorb and separate heavy metals and other problems, and achieve good heavy metal desorption and regeneration performance, good desorption and desorption performance, and excellent adsorption effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
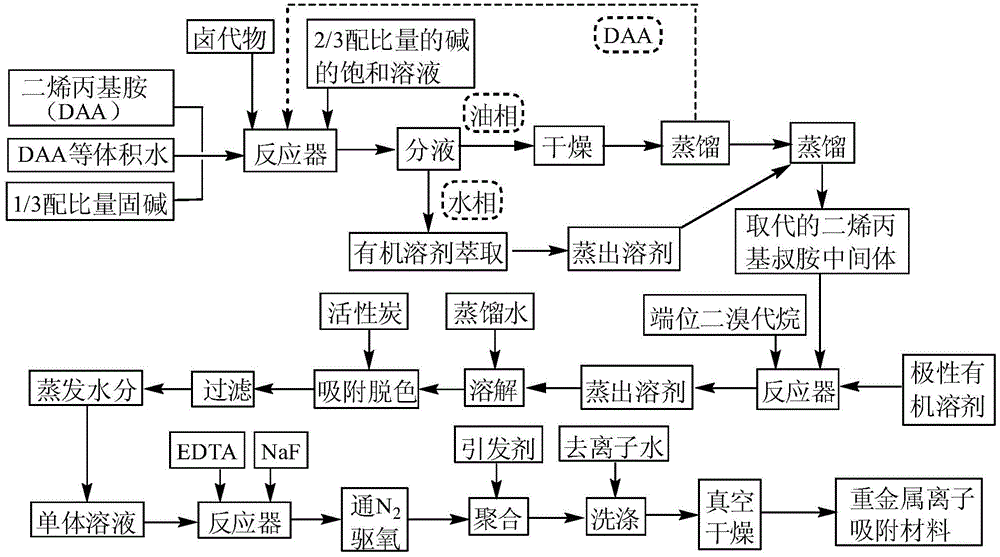
[0032] (1) Mix diallylamine, ethyl chloroacetate and potassium carbonate in a molar ratio of 1.2:1:1, first add 55.4mL of diallylamine and 55.4mL of water to a 500mL Add 17.02g of solid potassium carbonate to the dropping funnel and mechanically stirred reactor, raise the temperature to 50°C, slowly add 40mL of ethyl chloroacetate dropwise, finish dropping within 3 hours, then add 65.3g of potassium carbonate saturated solution dropwise, within 2h After adding, continue to react for 2h;
[0033] (2) Transfer the above liquid phase into a separatory funnel, let it stand for 30 minutes, separate the liquids, extract the water phase with ether for 3 times, combine the extracts, and distill off the solvent to obtain 5.1 mL of residue; Potassium drying, distill 8.5mL of unreacted diallylamine into the next round of reaction, combine the distillate with the extracted distillate, distill under reduced pressure to -0.093MPa, collect 56.4mL of distillate at 126~130℃, That is, the subs...
Embodiment 2
[0039] (1) Mix diallylamine, ethyl chloroacetate and potassium carbonate in a molar ratio of 1.6:1:1.02, first add 73.9mL of diallylamine and 73.9mL of water to a 500mL Add 17.37g of solid potassium carbonate to the dropping funnel and mechanically stirred reactor, raise the temperature to 60°C, slowly add 40mL of ethyl chloroacetate dropwise, finish dropping within 3 hours, then add 66.62g of saturated potassium carbonate solution dropwise, within 2h After adding, continue to react for 2h;
[0040] (2) Transfer the above liquid phase into a separatory funnel, let it stand for 30 minutes, separate the liquids, extract the water phase twice with ethyl acetate, combine the extracts, and distill off the solvent to obtain 5.4 mL of distilled residue; Water was dried with potassium carbonate, and 25.2 mL of unreacted diallylamine was distilled off to enter the next round of reaction. The distillate was combined with the extracted distillate, distilled under reduced pressure to -0.0...
Embodiment 3
[0044] (1) Mix diallylamine, ethyl chloroacetate and potassium carbonate in a molar ratio of 2.0:1:1.05, first add 92.4mL of diallylamine and 92.4mL of water to a 500mL Add 17.88g of solid potassium carbonate to the dropping funnel and mechanically stirred reactor, raise the temperature to 70°C, slowly add 40mL of ethyl chloroacetate dropwise, finish dropping within 3 hours, then add dropwise 68.57g of potassium carbonate saturated solution, within 2h After adding, continue to react for 2h;
[0045] (2) Transfer the above liquid phase into a separatory funnel, let it stand for 30 minutes, separate the liquids, extract the water phase with ethyl acetate for 3 times, combine the extracts, and evaporate the solvent to obtain 6.7mL distillate; Water was dried with potassium carbonate, and 44.9 mL of unreacted diallylamine was distilled off to enter the next round of reaction; the distilled residue was combined with the extracted distilled residue, distilled under reduced pressure ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com