A kind of preparation method of bisfluorosulfonimide salt
The technology of a bisfluorosulfonimide salt and a purification method is applied in the field of preparation of the bisfluorosulfonimide salt, which can solve the problems of unobtainable reaction raw materials, low purity, difficulty in separation and purification, etc., and meets the requirements of operation and equipment The conditions are not harsh, the reaction steps are simple, and the effect of separation and purification is easy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Add 383g of acetonitrile and 130g of lithium carbonate to a 2L autoclave, cool the autoclave to -34°C after mixing, add 10g of anhydrous liquid NH 3 , slowly add 100g sulfuryl fluoride (F 2 SO 2 ) mixed to obtain a mixture; NH 3 , F 2 SO 2 The mol ratio with lithium carbonate is 1:2:3;
[0058] Heat the autoclave to 120°C, and continue to stir and reflux for 72 hours;
[0059] Cool to normal temperature then, filter to obtain filtrate;
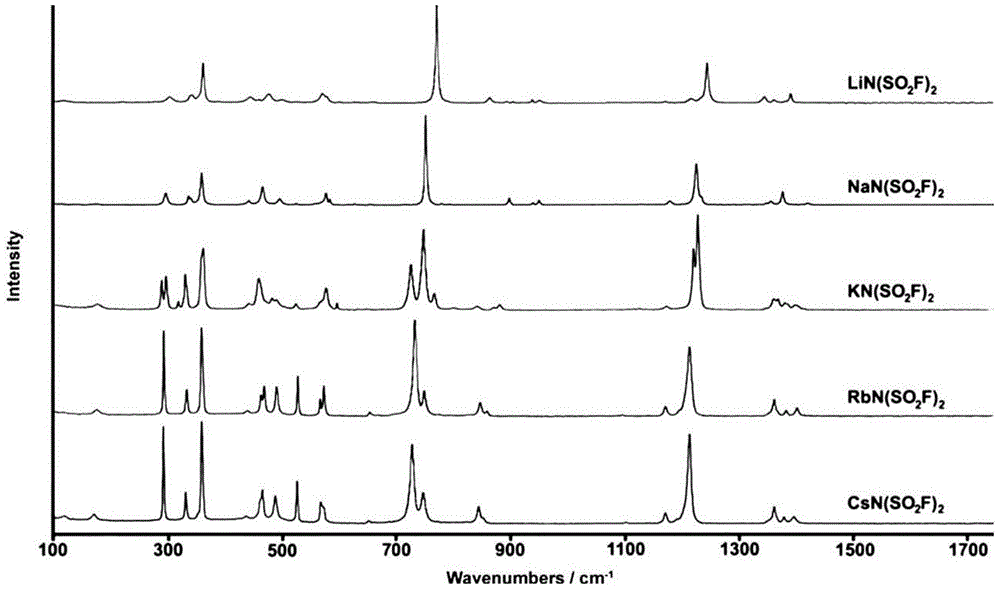
[0060] The solvent in the filtrate was evaporated and removed with a rotary evaporator to obtain a crude product. The crude product was put into an oven to be heated and vacuum-dried to obtain the final product. The final product was tested by Raman spectroscopy. The test conditions were: light source Nd:YAG ( λ=1064nm), the measured Raman spectrum is as follows figure 1 As shown, it is proved that the final product obtained is lithium bisfluorosulfonyl imide (LiN(SO 2 F) 2 ), heavy 98g, productive rate 89%.
[0061] The final...
Embodiment 2
[0064] Add 1000g of acetone and 420g of sodium bicarbonate to a 5L autoclave, cool the autoclave to -100°C after mixing, add 10g of anhydrous gaseous NH 3 , while stirring, slowly add 580g chlorosulfuryl fluoride (FSO 2 Cl) mixed to obtain a mixture; NH 3 、FSO 2 The molar ratio of Cl to sodium bicarbonate is 1:10:10;
[0065] Heat the autoclave to -50°C, and continue to stir and reflux for 48 hours;
[0066] Place to normal temperature then, filter to obtain filtrate;
[0067] The solvent in the filtrate was evaporated and removed with a rotary evaporator to obtain a crude product. The crude product was put into an oven to be heated and vacuum-dried to obtain the final product. The final product was tested by Raman spectroscopy. The test conditions were: light source Nd:YAG ( λ=1064nm), the measured Raman spectrum is as follows figure 1 As shown, it is proved that the final product obtained is sodium bisfluorosulfonyl imide (NaN(SO 2 F) 2 ), weighs 250.5g, and the yield...
Embodiment 3
[0069] Add 500g methylene chloride and 4245g potassium phosphate in 10L autoclave, after mixing, add 53.5g ammonium chloride, slowly add 152g bromosulfuryl fluoride (FSO 2 Br) mixed to obtain a mixture; ammonium chloride, FSO 2 The molar ratio of Br to potassium phosphate is 1:2:10;
[0070] Heat the autoclave to 50°C, and continue to stir and reflux for 5 hours;
[0071] Then cool and filter to normal temperature, filter to obtain the filtrate;
[0072] The solvent in the filtrate was evaporated and removed with a rotary evaporator to obtain a crude product. The crude product was put into an oven to be heated and vacuum-dried to obtain the final product. The final product was tested by Raman spectroscopy. The test conditions were: light source Nd:YAG ( λ=1064nm), the measured Raman spectrum is as follows figure 1 As shown, it is proved that the final product obtained is potassium bisfluorosulfonyl imide (KN(SO 2 F) 2 ), weighs 174.5g, and the yield is 86%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com