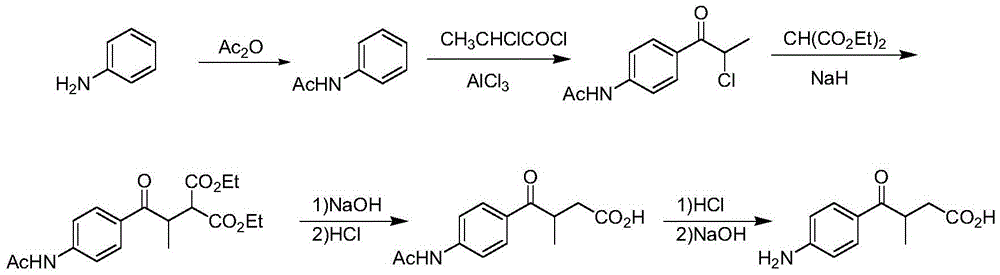
A kind of preparation method of 3-methyl-4-oxo-4-(p-amino)phenylbutyric acid
A technology of phenylbutyric acid and p-amino group is applied in the field of preparation of 3-methyl-4-oxo-4-(p-amino)phenylbutyric acid, and can solve the problems of high cost of elimination, complicated post-treatment process, The problem of high cost of raw materials, to achieve the effect of safe and simple operation, novel process design, and environmentally friendly reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
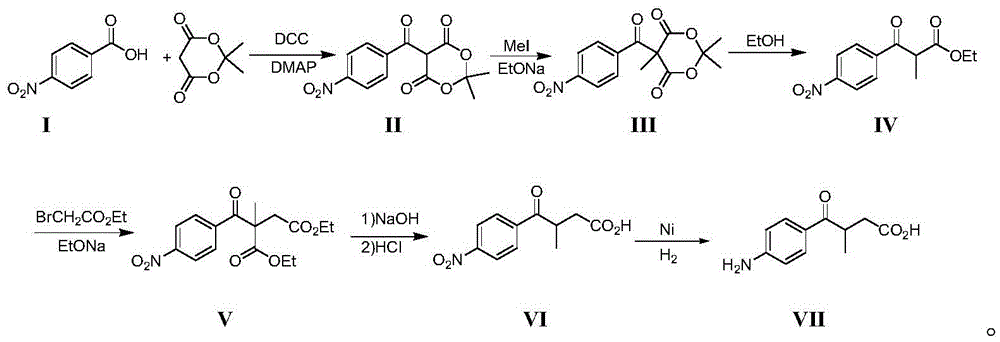
[0033] In the p-nitrobenzoic acid (I) of 500g, add the dichloromethane of 2.7kg, then successively add the propylidene malonate of 435g, the 4-N of 550g, N-dimethylaminopyridinium and 1.06kg of bicyclic Hexylcarbodiimide, the reaction temperature is 10°C, after 36 hours of reaction, filter out the insoluble solids, add 420 ml of 1N hydrochloric acid to the organic phase, stir well, and dry the separated organic phase with sodium sulfate for 5 hours , Sodium sulfate was filtered off, and it was suggested to evaporate the organic solvent to obtain 855kg of intermediate (II).
[0034] Add 2200g of DMF to 855g of intermediate (II), control the temperature of the mixture to 0°C in an ice-water bath, then add 200g of sodium ethylate to the mixture, then control the temperature of the reaction solution at -5°C, and add 460g of Methyl iodide was dropped in about 1 hour, and the reaction temperature was -5°C. After 13 hours of reaction, DMF was evaporated under reduced pressure, and th...
Embodiment 2
[0040] Add 3kg of dichloromethane in the p-nitrobenzoic acid (I) of 600g, then successively add 530kg of propylidene malonate, 520g of 4-N, N-dimethylaminopyridinamine and 1500g of dicyclohexyl carbon Diimine, the reaction temperature is 20°C, after reacting for 16 hours, filter out the insoluble solids, add 5 liters of 1N hydrochloric acid to the organic phase, stir well, dry the separated organic phase with sodium sulfate for 4 hours, filter Sodium sulfate was removed, and the organic solvent was suggested to be evaporated to obtain 1033g of intermediate (II).
[0041] Add 2.4kg of DMF to 1033g of intermediate (II), control the temperature of the mixed solution in an ice-water bath to 20°C, then add 290g of sodium ethylate to the mixed solution, then control the temperature of the reaction solution at 0°C, and add 540g of iodomethane, drop it in about 1 hour, and the reaction temperature is 5°C. After 10 hours of reaction, distill off DMF under reduced pressure, then add met...
Embodiment 3
[0047] In the p-nitrobenzoic acid (I) of 3kg, add the dichloromethane of 15kg, then successively add the propylidene malonate of 2.6kg, the 4-N of 3.1kg, N-dimethylaminopyridinamine and 7.4kg of dichloromethane Cyclohexylcarbodiimide, the reaction temperature is 28°C, after 32 hours of reaction, filter out the insoluble solids, add 25 liters of 1N hydrochloric acid to the organic phase, stir well, and dry the separated organic phase with sodium sulfate for 5 hours Finally, sodium sulfate was filtered off, and the organic solvent was suggested to be evaporated to obtain 5.15kg of intermediate (II).
[0048] Add 12kg of DMF to 5.15kg of intermediate (II), control the temperature of the mixed solution to 10°C in an ice-water bath, then add 1.44kg of sodium ethylate to the mixed solution, then control the temperature of the reaction solution at -5°C, drop Add 2.74kg of methyl iodide, drop it for about 1 hour, and the reaction temperature is 0°C. After 12 hours of reaction, distill...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com