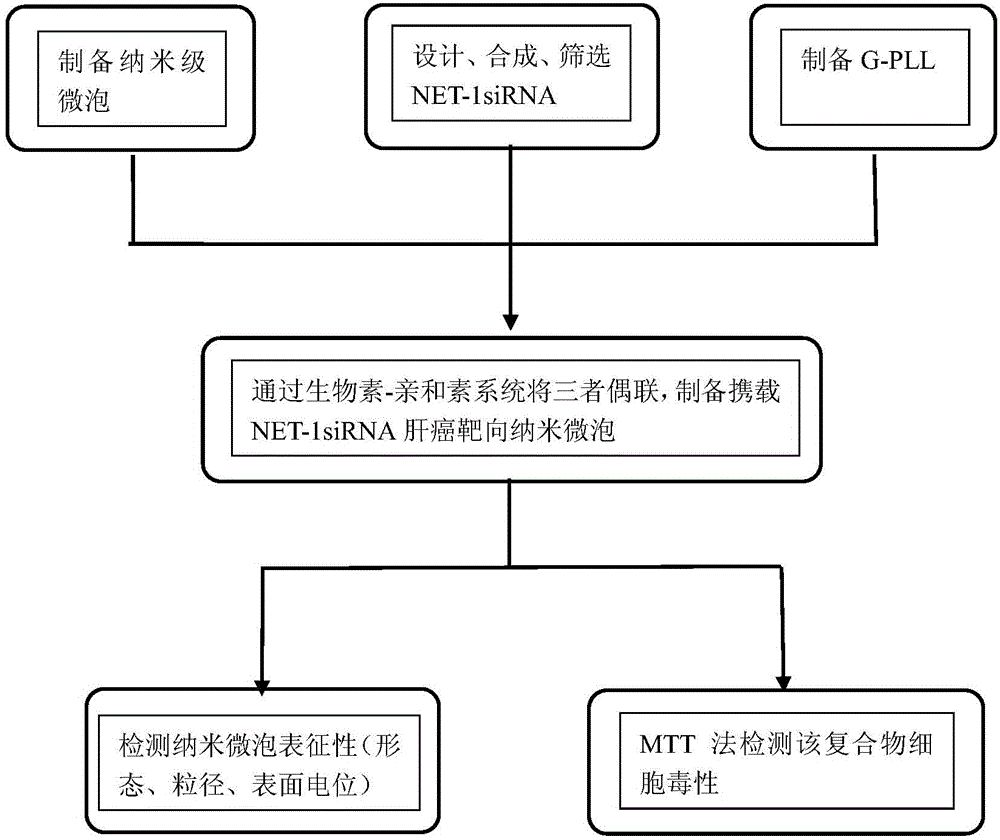
Preparation method of difunctional liver cancer targeted nanobubble carrying NET-1siRNA
A dual-function, microbubble technology, applied in the field of biomedical engineering, can solve the problems of poor tumor tissue specificity and inability to guarantee siRNA concentration, and achieve the effects of improving gene transfection rate, inhibiting the growth of liver cancer cells, and improving transfection rate.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Embodiment 1 design, synthesis, screening effective NET-1siRNA
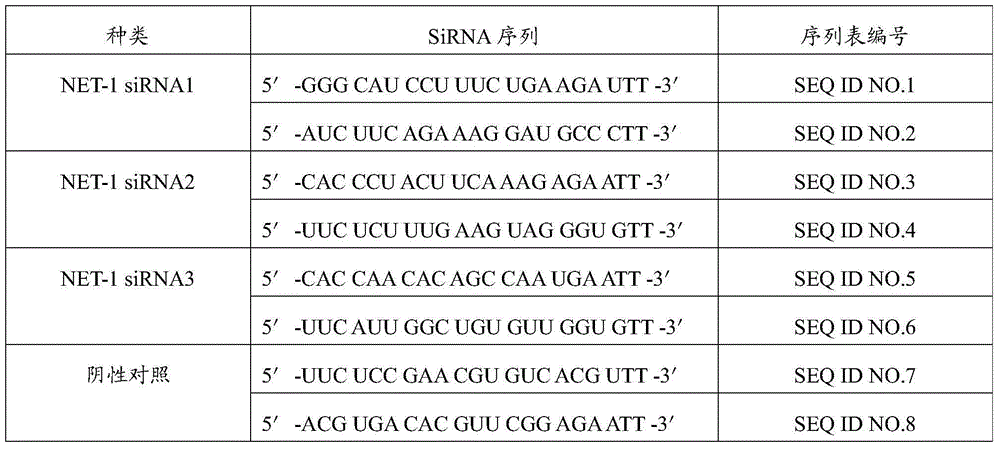
[0040] According to the gene sequence of human NET-1 mRNA, according to the basic design principles of siRNA, select 3 target sequences, design 3 pairs of NET-1 siRNA, and design an irrelevant control sequence: negative control siRNA (Table 1, provided by Shanghai Gemma Pharmaceutical Technology Co., Ltd. synthesis). Three pairs of NET-1 and negative control siRNA were labeled with FAM fluorescence and biotin, freeze-dried and stored at -20°C.
[0041] Table 1 Sequences of three NET-1 siRNAs and negative controls
[0042]
Embodiment 2
[0043] Example 2 Preparation of Biotinylated Nanobubbles (Nanobubble)
[0044] 10 mg of lipid mixture (mass ratio, DSPC: DSPE-PEG2000: DSPE-PEG2000-biotin=9:0.5:0.5) was placed in a round bottom flask, and a mixed solution of chloroform and methanol was added dropwise (volume ratio, chloroform:methanol= 2:1), until the lipid mixture was completely dissolved. Place the flask in a rotary evaporator and evaporate to dryness at 40°C under vacuum;
[0045] After evaporating to dryness, add 5 mL of PBS (pH=7.4, 0.15 mol / L) buffer solution in the flask, pipette it into a centrifuge tube, seal it, and replace the internal air with C 3 f 8 ;
[0046] The amalgam oscillator was shaken for 40 seconds, and then the solution was placed under an ultrasonic breaker, in a water bath for 20 seconds, and the temperature was 55°C. Stop breaking, incubate at 55°C for 60 minutes, and then continue breaking for 2 minutes. After standing still, the microspheres in the upper layer are floated, w...
Embodiment 3
[0047] Example 3 Synthesis of biotinylated galactosylated polylysine (Bio-G-PLL)
[0048] Weigh 113.2 mg of polylysine, 480.5 mg of galactose, and 300.0 mg of sodium borohydride and dissolve them in 120 mL of water, place them in a round-bottomed flask, add a stirrer, and react at a constant temperature of 37 ° C for 24 hours. Potassium hydroxide was used to adjust the pH of the reaction solution to 8.5, and the reaction was continued at 37°C for 6 hours.
[0049] Dialysis bag pretreatment: After cutting the dialysis bag to an appropriate length (about 30cm), use NaHCO with a mass fraction of 2% 3 (M / V) and 10mmol / L EDTA (pH8.0) were boiled for 10min, then cleaned with distilled water, put in 1mmol / L EDTA (pH8.0) and boiled for 10min, cooled, stored at 4°C, guaranteed bag Submerge completely in solution. Fill the bag with clean water before use, and use it after draining.
[0050] Dialysis process: put the reaction solution into a dialysis bag, tie both ends with a thin thr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com