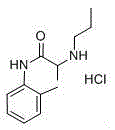
Method for preparing propitocaine hydrochloride
A technology for prilocaine hydrochloride and n-propylamine, applied in the field of preparation of prilocaine hydrochloride, can solve the problems of reducing product yield, tedious operation, flammable solvent, etc., and achieves reduction of production cost, tedious operation, and shortened reaction time. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
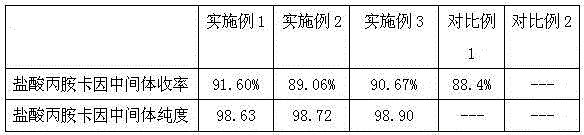
[0043] (a) Add 107g o-toluidine in 600g methylene chloride, stir at room temperature in a 1000ml reaction flask, the rotating speed is 80 revolutions per minute, drop 139g α-chloropropionyl chloride at room temperature, the rate of addition is 4.5 per minute g, the dropping time is 30min. After dropping α-chloropropionyl chloride, the reaction solution is incubated at 20°C for 2.5h. After the incubation is completed, the reaction solution is washed with 4% hydrochloric acid, and the upper layer is discarded. Wash with 3.5% aqueous sodium carbonate solution, discard the upper layer, add 240% water to the organic layer, stir and separate out off-white solid, filter, dry to obtain prilocaine hydrochloride intermediate 181g, yield 93.0%, according to high performance liquid chromatography (HPLC ) measurement, its prilocaine hydrochloride intermediate purity 98.63%.
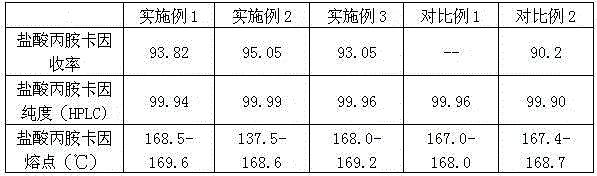
[0044] (b) Add 100g of prilocaine hydrochloride intermediate to 100g of n-propylamine solution, heat to reflux for ...
Embodiment 2
[0046] (a) add 107g o-toluidine in 800g dichloromethane, stir at room temperature in the reaction flask of 1000ml, the rotating speed is 80 revolutions per minute, drip 128.5g α-chloropropionyl chloride under the condition of room temperature, the rate of addition per minute is 4.5g, the dropping time is 30min. After dropping α-chloropropionyl chloride, the reaction solution is kept at 22°C for 2 hours. Wash with 5% aqueous sodium carbonate solution, discard the upper layer, add 220g of water to the organic layer, stir and precipitate an off-white solid, filter, and dry to obtain 176g of prilocaine hydrochloride intermediate, yield: 89.06%, according to high performance liquid chromatography Method (HPLC) measures, and its prilocaine hydrochloride intermediate purity 98.72%.
[0047] (b) Add 100g of prilocaine hydrochloride intermediate to 100g of n-propylamine solution, heat to reflux for 7h, after the reaction is finished, add concentrated hydrochloric acid to adjust pH=1-2,...
Embodiment 3
[0049] (a) Add 107g o-toluidine in 750g methylene chloride, stir at room temperature in a 1000ml reaction flask, the rotating speed is 80 revolutions per minute, drop 142g α-chloropropionyl chloride at room temperature, the rate of addition is 4.5 per minute g, the dropping time is 30min. After dropping α-chloropropionyl chloride, the reaction solution is incubated at 18°C for 3 hours. After the incubation is completed, the reaction solution is washed with 5.5% hydrochloric acid, the upper layer is discarded, the organic layer is retained, and the organic layer is reused. Wash with 4% aqueous sodium carbonate solution, discard the upper layer, add 160% water to the organic layer, stir and precipitate an off-white solid, filter, and dry to obtain 179.2g of prilocaine hydrochloride intermediate, yield: 90.67%, according to high performance liquid chromatography (HPLC) measures, and its prilocaine hydrochloride intermediate purity 98.90%.
[0050] (b) Add 100g of prilocaine hyd...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com