A method of adding disulfide bonds to improve the thermostability of ferulic acid esterase a
A technology of ferulic acid esterase and nucleotide, applied in the field of bioengineering, can solve the problems of poor thermal stability of natural ferulic acid esterase, achieve large industrial production and application potential and economic value, improve thermal stability, The effect of improving thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
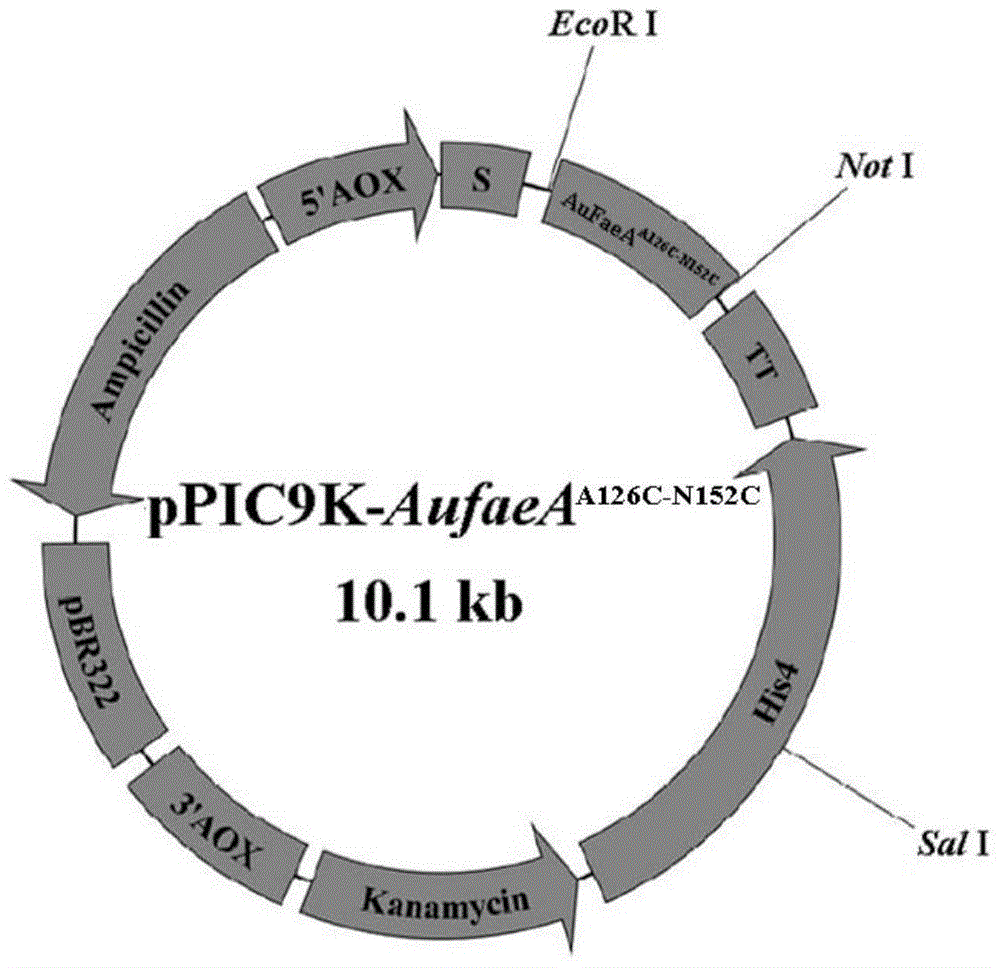
[0020] Example 1 Mutant gene AufaeA A126C-N152C and its expression plasmid construction
[0021] Using large primer PCR technology to construct the fusion gene, it is mainly divided into four steps: use 126-F, 152-R as primers for the first round of PCR (94°C for 5min; 2 cycles, 94°C for 30s, 45°C for 30s, 72°C for 15s ; 28 cycles, 94°C for 30s, 55°C for 30s, 72°C for 15s; 72°C for 10min; 10°C for storage) to obtain the gene fragment AufaeA 126-152 ; With pUCm-T-AufaeA as a template, the first round of PCR product AufaeA 126-152 Carry out the second round of large primer PCR (94°C for 5min; 2 cycles, 94°C for 30s, 45°C for 30s, 72°C for 30s) for the primer and the specific primer FAE-F in the applied invention patent (application number: 201210181372.X). 28 cycles, 94°C for 30s, 55°C for 30s, 72°C for 30s; 72°C for 10min; 10°C for preservation), the gene fragment AufaeA was obtained F-152 ; With pUCm-T-AufaeA as a template, the second round of PCR product AufaeA F-152 The ...
Embodiment 2
[0023] Embodiment 2GS115 / AufaeA A126C-N152C Construction, expression and determination of properties of recombinants
[0024] pPIC9K-AufaeA with SalI A126C-N152C Perform linearization, perform electrotransformation and screening according to the Pichia expression manual, and obtain high-copy Pichia recombinant GS115 / AufaeA A126C-N152C . The engineered bacteria were induced with 1.0% methanol for 72 hours. The centrifuged supernatant is the recombinant ferulic acid esterase A crude enzyme solution, which is detected as a single band by SDS-PAGE, and the recombinant AuFaeA is transformed A126C-N152C The optimum reaction temperature is 50°C, which is 5°C higher than that of the original enzyme. The half-life at 60°C is 40 minutes, and the thermal stability is also greatly improved compared with the original enzyme.
[0025]
[0026]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com