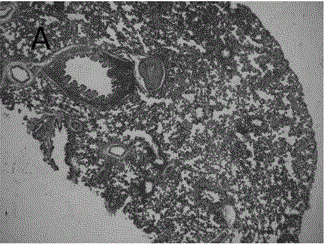
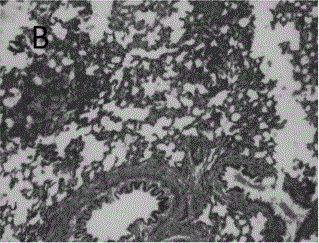
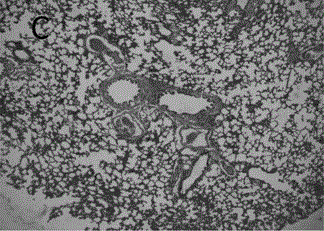
A kind of astragalus polysaccharide slow-release microspheres for treating radiation pneumonitis and preparation method thereof
A technology of radiation pneumonitis and astragalus polysaccharide, applied in the field of biomedicine, can solve the problems of lack of tissue and organ targeting, high local blood drug concentration, inability to reach the action time, etc., and achieve good mental state, rich blood flow, and prolonged absorption. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] An astragalus polysaccharide slow-release microsphere for treating radiation pneumonitis, the mass ratio of astragalus polysaccharide and chitosan is 1:3, and the polymerization degree of chitosan is 30CPS.
[0036] A preparation method for the astragalus polysaccharide slow-release microspheres for the treatment of radiation pneumonitis, comprising the following steps:
[0037] (1) Preparation of raw material solution
[0038] Accurately weigh 3 parts of chitosan with a degree of polymerization of 30CPS and 1 part of astragalus polysaccharide, which are vacuum-dried to constant weight at room temperature, dissolve in 1000 mL of 0.5% acetic acid solution, and mix;
[0039] (2) Filtration, spray drying
[0040] Filter the mixed solution obtained in the above step (1) through a 0.45 μm microporous membrane to obtain the filtrate. The obtained subsequent filtrate is introduced into the double-flow spiral nozzle of the Büchi 290 small spray dryer through a peristaltic pump...
Embodiment 2
[0044] An astragalus polysaccharide slow-release microsphere for treating radiation pneumonitis, the mass ratio of astragalus polysaccharide and chitosan is 1:5, and the polymerization degree of chitosan is 50CPS.
[0045] A preparation method for the astragalus polysaccharide slow-release microspheres for the treatment of radiation pneumonitis, comprising the following steps:
[0046] (1) Preparation of raw material solution
[0047]Accurately weigh 5 parts of chitosan with a degree of polymerization of 50CPS and 1 part of astragalus polysaccharide, which were vacuum-dried to constant weight at room temperature, dissolved in 1000 mL of 0.5% acetic acid solution, and mixed;
[0048] (2) Filtration, spray drying
[0049] Filter the mixed solution obtained in the above step (1) through a 0.45 μm microporous membrane to obtain the filtrate. The obtained subsequent filtrate is introduced into the double-flow spiral nozzle of the Büchi 290 small spray dryer through a peristaltic p...
Embodiment 3
[0053] An astragalus polysaccharide slow-release microsphere for treating radiation pneumonitis, the mass ratio of astragalus polysaccharide and chitosan is 1:7.
[0054] A preparation method for the astragalus polysaccharide slow-release microspheres for the treatment of radiation pneumonitis, comprising the following steps:
[0055] (1) Preparation of raw material solution
[0056] Accurately weigh 5 parts of chitosan with a degree of polymerization of 130CPS, 2 parts of chitosan at 50CPS, and 1 part of astragalus polysaccharide that were vacuum-dried to constant weight at room temperature, dissolved in 1000 mL of 0.5% acetic acid solution, and mixed;
[0057] (2) Filtration, spray drying
[0058] Filter the mixed solution obtained in the above step (1) through a 0.45 μm microporous membrane to obtain the filtrate. The obtained subsequent filtrate is introduced into the double-flow spiral nozzle of the Büchi 290 small spray dryer through a peristaltic pump, and the inlet te...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of polymerization | aaaaa | aaaaa |
| encapsulation rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com