traditional Chinese medicine composition for treating cardiovascnlar and cerebrovascular diseases
A technology of cardiovascular and cerebrovascular diseases and compositions, applied in the field of traditional Chinese medicine compositions, can solve problems such as patients’ resistance to taking medicines, sores or numbness of limbs, stomach discomfort, etc., to improve cardiovascular and cerebrovascular circulation and reduce blood viscosity , dizziness improvement effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
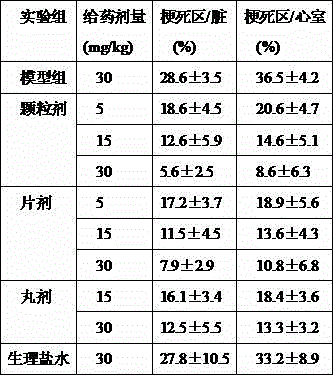
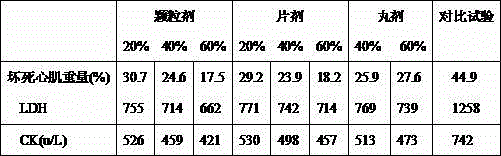
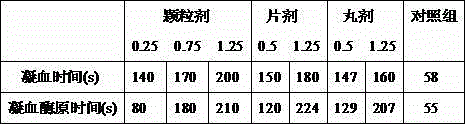
Examples
Embodiment 1
[0041] Preparation of pharmaceutical granules of the present invention
[0042] Chinese medicine composition raw material preparation:
[0043] Panax notoginseng extract: Use notoginseng in the winter of the third year after planting, remove the fibrous roots, dry it until the surface is free of moisture, then crush it into a drug powder with a particle size of 50-100um, and extract it with 70% (v / v) ethanol ultrasonically. times, the extraction time is 1.5h, 1.0h and 0.5h in sequence. After extraction, heat at 50~65°C to remove ethanol, and add the remaining extract to distilled water to dilute, so that the ethanol content in the dilution does not exceed 0.5%, and then use a hollow Fiber membrane filter is carried out ultrafiltration, wherein ultrafiltrate, concentration, macroporous adsorption resin column adsorption, water washing, 50% (v / v) ethanol elution, decompression concentration, vacuum drying obtains notoginseng saponin extract; After analysis Notoginsenoside R g1...
Embodiment 2
[0048] Embodiment 2 The preparation of medicine pill of the present invention
[0049] Preparation of traditional Chinese medicine composition:
[0050] Panax notoginseng extract: Use notoginseng in the summer of the third year after planting, remove the fibrous roots, dry it in the sun until the surface moisture content is 1%, and then crush it into the original drug powder with a particle size of 10~50um, and use 50% (v / v) ethanol Ultrasonic extraction 3 times, each extraction time is 1.5h, 0.8h and 0.5h, wherein the amount of ethanol is 28 times the weight of the original drug (10 times the weight of the original drug is added to ethanol for the first purification, and the original drug is added for the second purification) 8 times of the weight of the medicine, ethanol of 10 times of the weight of the original medicine is added for the third purification), wherein the extraction temperature is 60°C; after extraction, the rotary evaporation removes the ethanol, so that ther...
Embodiment 3
[0055] Embodiment 3 Preparation of pharmaceutical tablet of the present invention
[0056] Raw material preparation of traditional Chinese medicine composition
[0057] Panax notoginseng extract: Use notoginseng after autumn in the third year after planting, wash and dry to make the surface moisture content of notoginseng 0.5%, and then crush it into the original drug powder with a particle size of 10~20um, use 50% (v / v) Ultrasonic extraction of ethanol for 3 times, each extraction time is 1.5h, 1.2h and 0.4h, wherein the amount of ethanol is 23 times of the weight of the original drug (10 times of the weight of the original drug is added to ethanol for the first purification, the second time Purify by adding 5 times of ethanol of the original drug weight, the third ethanol dosage is 8 times of the original drug), wherein the extraction temperature is 70 °C; after extraction, the rotary evaporation removes ethanol, so that the ethanol content in the extract is 0.2%, and then ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com