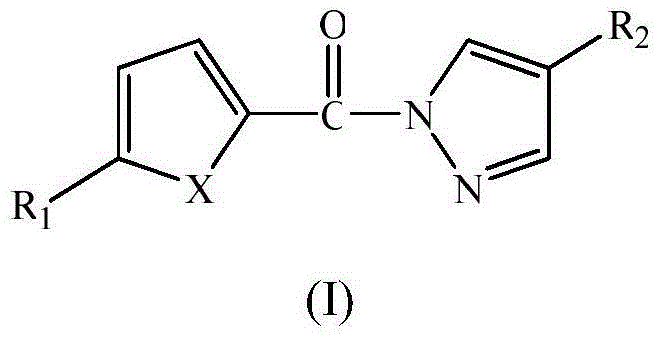
Pyrazole amide compounds and application thereof
A technology of pyrazole amide and compound, which is applied in the field of pyrazole amide compounds and their application as agricultural fungicides, can solve the problems of no pyrazole amide compounds, etc., and achieve simple structure, low cost and good control effect Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] compound (C 8 h 6 N 2 OS) preparation
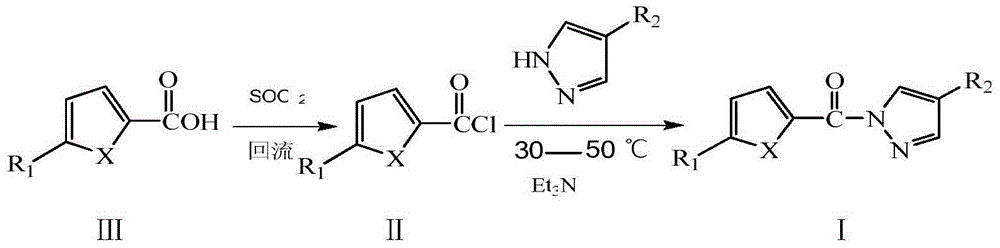
[0030] (1) Add 0.01mol thiophene-2-carboxylic acid and 15mL thionyl chloride to a 50mL three-neck flask, heat to 80°C and reflux for 2 hours, and distill off excess thionyl chloride until no liquid flows out, Cool to room temperature to obtain thiophene-2-formyl chloride.
[0031] (2) Add 0.01mol pyrazole and 10mL dichloromethane into a 50mL three-neck flask, add 3mL triethylamine, and add fresh thiophene-2-formyl chloride dropwise under stirring at room temperature. After the dropwise addition, continue the reaction at 30-50°C for 3 hours. After the reaction is complete, wash the reaction mixture with 10% hydrochloric acid, 10% NaOH and distilled water to neutrality, and place it in a refrigerator at 2-6°C until the solid precipitates Afterwards, filter, wash, and recrystallize with dimethyl sulfoxide and water to obtain a milky white crystal product with a yield of 54%. The spectral data of the product are as follows:
...
Embodiment 2
[0035] compound (C 8 h 5 N 2 o 2 Br) preparation
[0036] (1) Add 0.01mol of furan-2-carboxylic acid and 15mL of thionyl chloride to a 50mL three-necked flask, heat to 80°C and reflux for 2 hours, distill off excess thionyl chloride until no liquid flows out, Cool to room temperature to obtain furan-2-formyl chloride.
[0037] (2) Add 0.01mol 4-bromopyrazole and 10mL dichloromethane into a 50mL three-necked flask, add 3mL pyridine, and add the newly prepared furan-2-formyl chloride dropwise under stirring at room temperature. After the dropwise addition, continue to react at 30-50°C for 2 hours. After the reaction is completed, wash the reaction mixture with 10% hydrochloric acid, 10% NaOH solution and distilled water to neutrality, and place it in a refrigerator at 2-6°C until solid After precipitation, filter, wash, and recrystallize with dimethyl sulfoxide and water to obtain a white crystal product with a yield of 55%. The spectral data of the product are as follow...
Embodiment 3
[0042] compound (C 8 h 5 N 2 OSBr) preparation
[0043] (1) Add 0.01mol thiophene-2-carboxylic acid and 15mL thionyl chloride to a 50mL three-neck flask, heat to 80°C and reflux for 2 hours, and distill off excess thionyl chloride until no liquid flows out, Cool to room temperature to obtain thiophene-2-formyl chloride.
[0044] (2) Add 0.01mol 4-bromopyrazole and 10mL dichloromethane into a 50mL three-necked flask, add 3mL triethylamine, and add the newly prepared thiophene-2-formyl chloride dropwise under stirring at room temperature. After the dropwise addition, continue the reaction at 30-50°C for 4 hours. After the reaction is complete, wash the reaction mixture with 10% hydrochloric acid, 10% NaOH and distilled water to neutrality, and place it in a refrigerator at 2-6°C until the solid precipitates Afterwards, filter, wash, and recrystallize with dimethyl sulfoxide and water to obtain a milky white crystal product with a yield of 67%. The spectral data of the pro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com