Caffeoyl hydroquinone ester and preparation method and application of caffeoyl hydroquinone ester in preparation of tyrosinase inhibitor
A technology of tyrosinase and inhibitors, applied in the field of a new natural compound of caffeoyl p-hydroxyphenol ester, which can solve pigmentation, dark spots, age spots, reduce storage period, nutritional value and market value, pigmentation Diseases and other problems, to achieve the effect of wide application potential and rich material sources
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
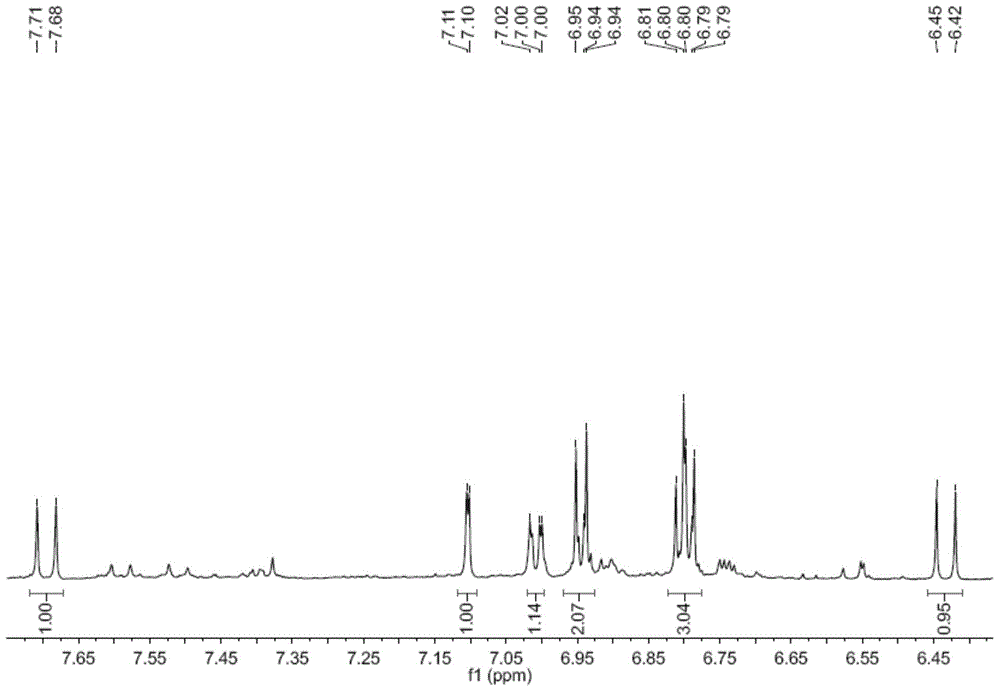
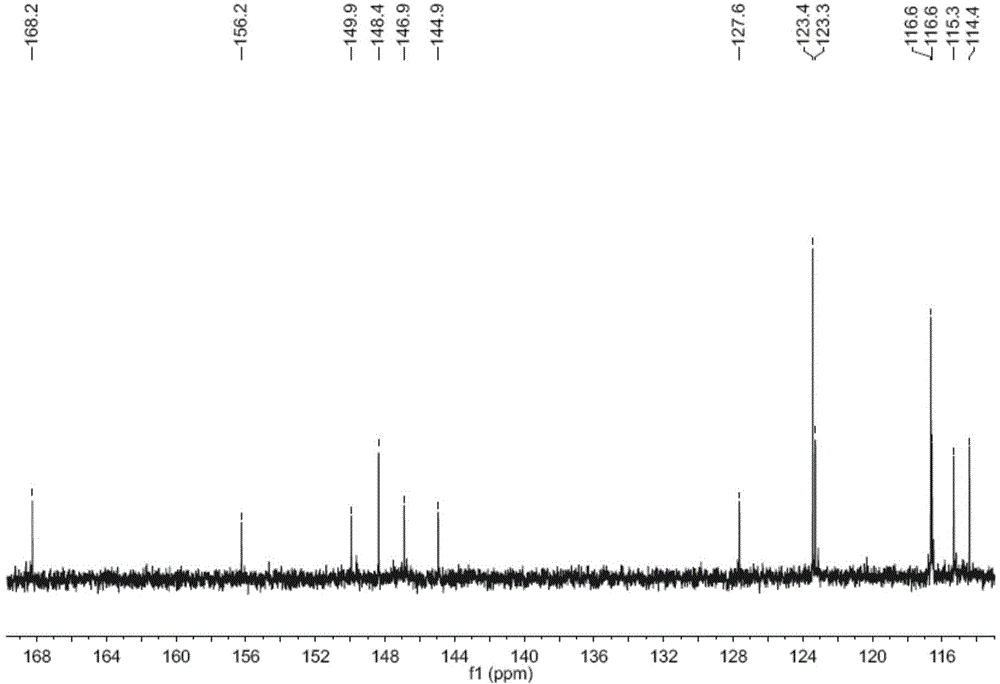
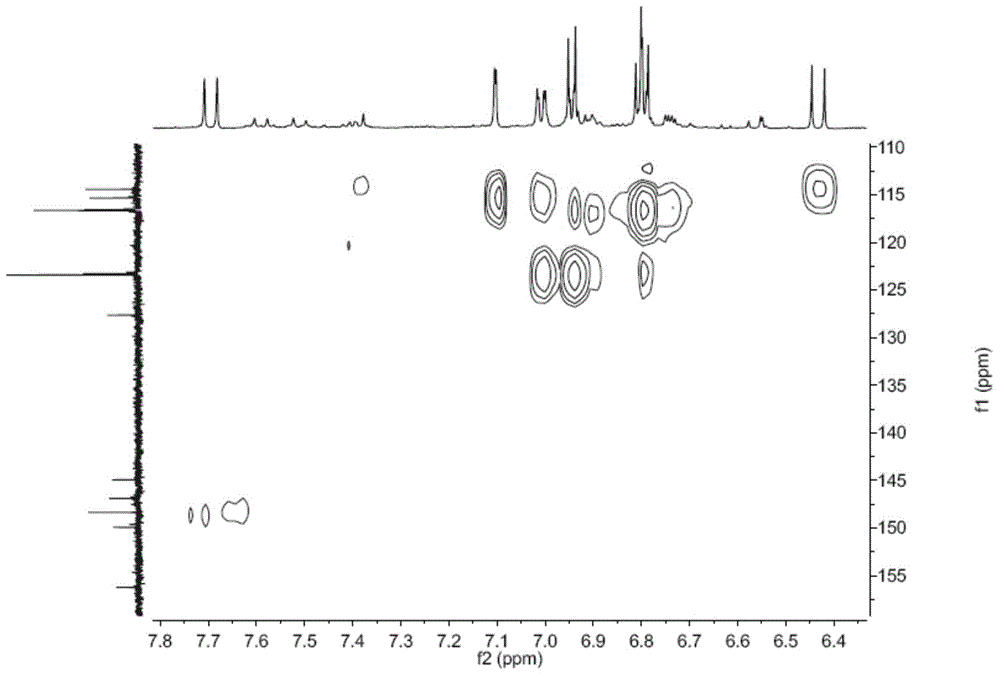
[0028] Example 1: Preparation of caffeoyl p-hydroxyphenol ester p-hydroxyphenyl caffeate in Wedelia chinensis
[0029] 1.1 Instruments and reagents
[0030] The decompression concentration adopts N-1000 rotary evaporator, CCA-1110 circulating cooling box and SB-1000 electric heating constant temperature water bath from Tokyo Physical and Chemical Company of Japan; instrument and C18 reverse-phase chromatographic column (particle size 50 μm, YMC Co. Ltd., Kyoto, Japan, 400 mm × 25 mm); electrospray mass spectrometry (ESIMS) using MDS SCIEX API 2000 LC / MS / MS instrument from Applied Biosystems, USA, with Methanol is used as a solvent for direct sampling determination; 1 H NMR spectrum and 13 C NMR spectrum was determined by Bruker AVANCE 600 nuclear magnetic resonance instrument, and tetramethylsilane was used as internal standard. The color development method is treated with 10% sulfuric acid ethanol solution and then heated for color development.
[0031] 1.2 Plant source a...
Embodiment 2
[0042] Example 2: Detection of tyrosinase inhibitory activity of compound p-hydroxyphenyl caffeate
[0043] 2.1 Reagents and instruments
[0044] Reagents: Tyrosinase was purchased from Sigma Chemical Co. (Sigma-Aldrich, St.Louis, USA), levodopa (L-DOPA) and kojic acid were purchased from Aladdin Industrial Corporation (USA), Na 2 HPO 4 , NaH 2 PO 4 , the compound to be tested was prepared for phytochemical analysis experiments;
[0045] Experimental equipment: microplate reader (Genois microplate reader, Tecan GENios, Switzerland).
[0046] 2.2 Test method
[0047] a) Preparation of drug solution: the compound to be tested, p-hydroxyphenyl caffeate, and kojic acid were formulated with dimethyl sulfoxide (DMSO) to form a 10 mg / ml solution, 67 mmol of phosphate buffer (prepared with ultrapure water), 46 U / ml Tyrosinase solution (prepared with phosphate buffer), 2.5mM L-DOPA (prepared with phosphate buffer).
[0048] b) Using a colorimetric method to complete the determin...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com