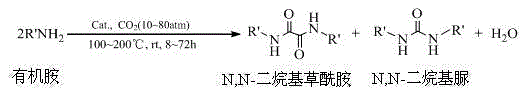
Method for synthesizing N,N-dialkyl oxamide by using CO2
A technology of dialkyl oxamide and dialkyl urea, which is applied in the field of synthesizing N-alkyl oxamide, and achieves a considerable yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] A use of CO 2 Preparation of N, the production method of N'-dibutyloxamide:
[0029] Take 10ml (0.103mol) of n-butylamine and put it into a stainless steel autoclave with 50ml of polytetrafluoroethylene lining and magnetic stirring, then add catalyst lanthanum tartrate 1.4g (0.005mol) and cocatalyst molecular sieve dehydrating agent 3g, organic solvent 1,3-Dimethyl-2-imidazolinone (DMI) 20ml, at room temperature, filled with 2.5MPa of CO 2 , seal the reaction vessel, raise the temperature to 150°C, and react at constant temperature for 24h. Cool, vent to normal pressure and normal temperature, take out the light yellow reaction liquid, filter the catalyst and dehydrating agent, and then distill off the inert solvent DMI under reduced pressure at about -0.096MPa and 180°C to obtain N,N'-dibutyl oxalamide, The vacuum distillation product was subjected to gas chromatography-mass spectrometry analysis, and the conversion rate of raw material n-butylamine was measured to b...
Embodiment 2
[0034] A use of CO 2 preparation N, N'- The production method of dihexyl oxalamide:
[0035] The operation is the same as in Example 1, and the solvent is the same as in Example 1. The organic amine n-butylamine is replaced by n-hexylamine, and the catalyst lanthanum tartrate is replaced by yttrium oxalate. When other conditions remain unchanged, the reaction temperature is 180 ° C, and the reaction time is 40 hours. The product N,N'-dihexyloxamide. The conversion rate of raw material n-hexylamine was measured to be 87%.
[0036] Product composition, selectivity and yield are shown in Table 2:
[0037] Table 2. Product composition, selectivity and yield
[0038] product composition Selectivity (%) Yield (%) N,N'-Dihexyloxamide 82.5 71.8 N,N'-Dihexylurea 16.3 14.2 Hexyl isocyanate 1.2 1.0
Embodiment 3
[0040] A use of CO 2 preparation N, N'- The production method of xylyl oxamide:
[0041] The operation is the same as in Example 1, the organic solvent DMI is replaced by diethylene glycol dimethyl ether (DME), n-butylamine is replaced by toluidine, the catalyst lanthanum tartrate is replaced by yttrium citrate, and the reaction temperature is At 180°C, the reaction time was 36 hours, and the product N,N'-xylyloxamide was obtained. The conversion rate of raw material toluidine is measured to be 40%.
[0042] Product composition, selectivity and yield are shown in Table 3:
[0043] Table 3. Product composition, selectivity and yield
[0044] product composition Selectivity (%) Yield (%) N,N'-xylyl oxamide 31.2 12.5 N,N'-Xylylurea 67.3 26.9 Tolyl isocyanate 1.5 0.6
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com