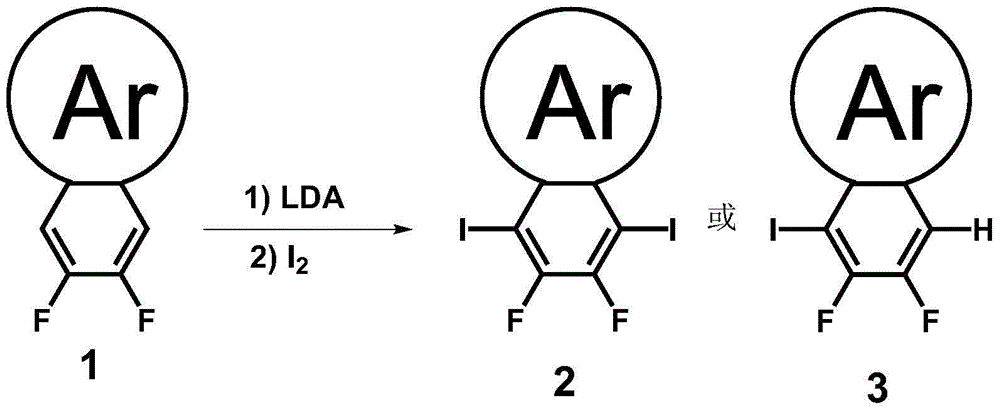
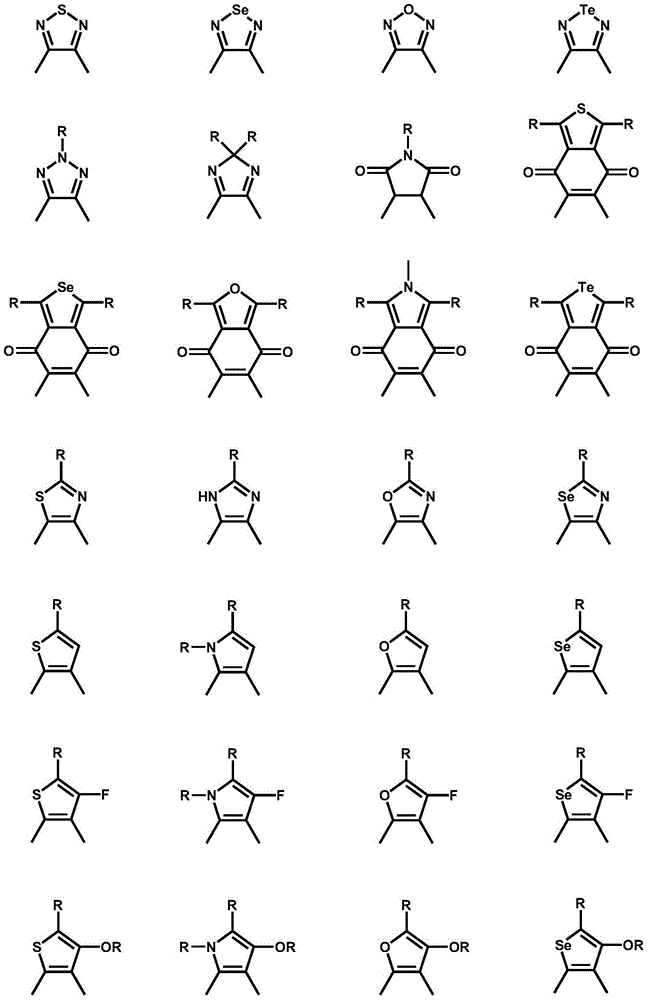
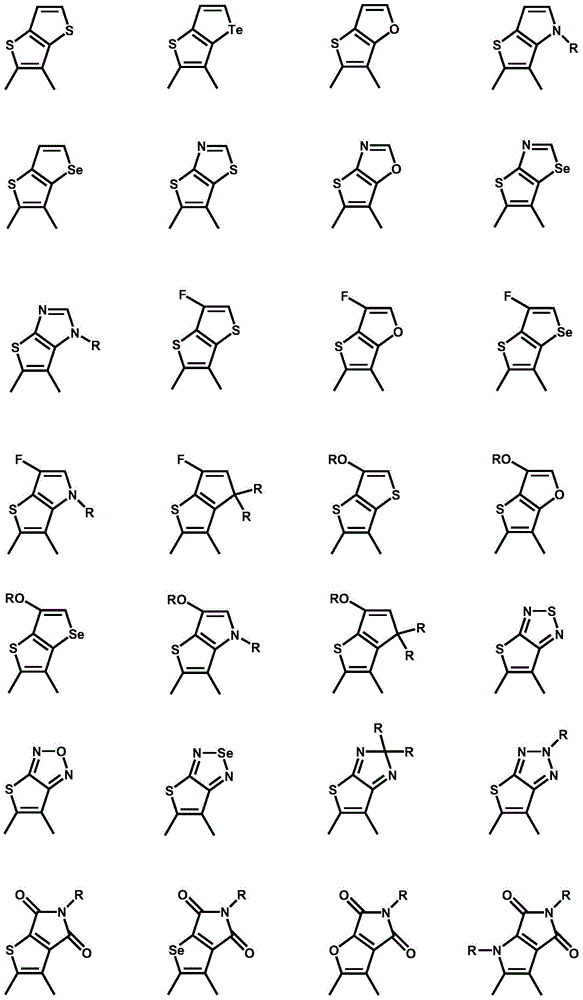
Preparation method of iodine atom-substituted bis-fluorophenyl heterocyclic conjugated monomer
A technology of conjugated monomers and difluorobenzene, which is applied in the field of fine organic chemistry, can solve problems such as potential safety hazards of experimental operators, achieve the effects of improving synthesis yield, facilitating large-scale preparation and production, and safe and reliable experimental operations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0023] (1) Preparation of Lithium Diisopropylamide (LDA): Under a nitrogen atmosphere, a solution of 0.1 g / mL diisopropylamine (1.1 equivalents) in tetrahydrofuran was cooled to -78°C, and 1 equivalent of n-butyl was slowly added dropwise to the solution. Lithium (2.5mol / L n-hexane solution), kept at -78°C for 1 hour after the dropwise addition.
[0024] (2) Iodination reaction: 1.0 equivalent of the substrate (difluorobenzoheterocyclic conjugated compound) was prepared into a 0.1g / L solution with anhydrous organic solvent, and then gradually added dropwise to LDA, at -78°C Under reaction 1 hour; The iodine simple substance of 2.3 equivalents (preparation of diiodine substitution monomer 2) or 1.15 equivalents (preparation of single iodine substitution monomer 3) and anhydrous organic solvent are made into the solution of 0.06g / L, and it is directly Added dropwise to the reaction system, the system naturally rose to room temperature, and stirred overnight.
[0025] (3) Post-p...
Embodiment 1
[0028] Preparation of 4,7-diiodo-2-dodecyl-5,6-difluoro-2H-benzo[d][1,2,3]triazole
[0029] The synthetic route is as follows:
[0030]
[0031] In a 250mL long-necked three-neck flask, under an argon atmosphere, add diisopropylamine (1.73g, 17.03mmol, 2.2eq) and 30mL of tetrahydrofuran, cool down to -78°C, add dropwise n-butyllithium (15.48mmol, 6.2mL, 2.5 mol / L, equivalent), completed, reacted for 1 hour, then 2-dodecyl-5,6 difluoro-2H-benzo[d][1,2,3]triazole (2.5g, 7.74mmol , 1 equivalent) and 25mL of tetrahydrofuran were made into a solution, and gradually added dropwise to the reaction solution. After completion of the reaction for 40 minutes, a tetrahydrofuran solution of iodine (4.52g, 17.8mmol, 2.3 equivalents, 75mL of tetrahydrofuran) was added dropwise thereto, and the reaction was completed. , stirred for 15 minutes, placed at room temperature, stirred overnight. Post-processing: add sodium sulfite and stir to remove iodine, then pour into water, extract with d...
Embodiment 2
[0033] Preparation of 4,7-diiodo-5,6-difluorobenzo[d][1,2,5]thiadiazole
[0034]
[0035]In a 250mL long-necked three-neck flask, under an argon atmosphere, add diisopropylamine (3.9g, 15.3mmol, 2.2eq) and 30mL of tetrahydrofuran, cool down to -78°C, add dropwise n-butyllithium (34.8mmol, 14.0mL, 2.5 mol / L, 2 equivalents), completed, reacted for 1 hour, and then 5,6 difluorobenzo[d][1,2,5]thiadiazole (3g, 17.4mmol, 1 equivalent) was compounded with 30mL of tetrahydrofuran Form a solution, gradually add dropwise to the reaction solution, complete, react for 40 minutes, then add iodine tetrahydrofuran solution (10.16g, 40.02mmol, 2.3 equivalents, 170mL tetrahydrofuran) dropwise therein, complete, after stirring for 15 minutes, place at room temperature , stirred overnight. Post-processing: add sodium sulfite and stir to remove iodine, then pour into water, extract with dichloromethane, wash with saturated aqueous sodium chloride, and dry over anhydrous magnesium sulfate. Th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com