Method for synthesizing acryloyl morpholine based on anhydride
A technology of acryloyl morpholine and morpholine, which is applied in the field of compound synthesis, can solve the problems of difficult control of the reaction process, poor stability of acryloyl chloride, and high corrosion of the reactor, so as to achieve easy control of the reaction, stable product quality, and low cost Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
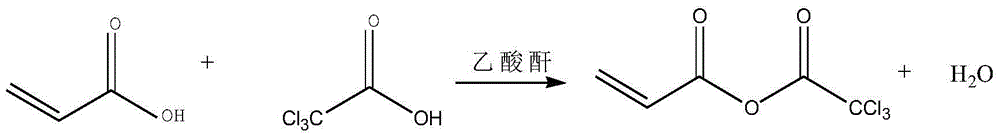
[0033] (1) Add 72g of acrylic acid and 164g of trichloroacetic acid in a four-necked flask to mix, add 5g of phosphorus pentoxide, add 2.6 grams of polymerization inhibitor, and add 110g of acetic anhydride dropwise to the above mixed solution. The reaction was carried out for 5 hours.
[0034] (2) Distill under reduced pressure after the completion of the reaction, remove the former fraction, and obtain 165 g of the product, which is acrylic acid trichloroacetic anhydride.
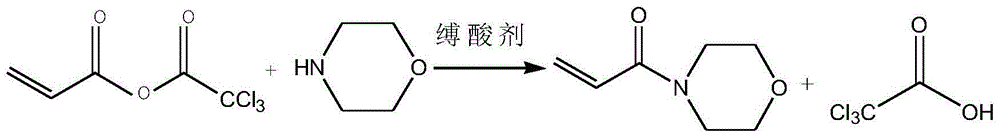
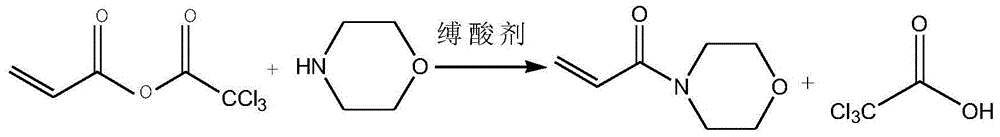
[0035] (3) Add 165g of acid anhydride into the solvent, lower the temperature to 0°C, add dropwise 72g of morpholine, and add 76.5g of triethylamine, keep the reaction temperature at 3°C, and finish the reaction for 2 hours to obtain crude acryloylmorpholine.
[0036] (4) Filtrate the crude acryloyl morpholine, add a polymerization inhibitor to distill under reduced pressure, collect fractions at 140-160° C., and obtain 96 g of the product, which is the final product acryloyl morpholine. The polymerizati...
Embodiment 2
[0039] (1) Add 900g of acrylic acid and 1025g of trichloroacetic acid in the reaction kettle to mix, add 50g of phosphorus pentoxide, add 26 grams of polymerization inhibitor, add 1100g of acetic anhydride to the above mixed solution dropwise, after the dropwise addition is completed, heat the mixture at 100°C Under the condition of reaction for 3 hours.
[0040] (2) Distill under reduced pressure after completion of the reaction to remove the front fraction to obtain 2071 g of acrylic acid trichloroacetic anhydride.
[0041] (3) Add 2071g of acid anhydride into the solvent, dropwise add 826.5g of morpholine, and add 682.5g of pyridine, keep the reaction temperature at 5°C, and react for 1 hour after the dropwise addition, to obtain crude acryloylmorpholine.
[0042] (4) Filtrate the crude acryloylmorpholine, add a polymerization inhibitor to distill under reduced pressure, collect fractions at 140-160°C, and obtain 1193 g of the product, which is the final product acryloylmor...
Embodiment 3
[0045] (1) Add 135g of acrylic acid and 615g of trichloroacetic acid in a four-necked flask to mix, add 10g of phosphorus pentoxide, add 5.2 grams of polymerization inhibitor, and add 200g of acetic anhydride dropwise to the above mixed solution. The reaction was carried out for 5 hours.
[0046] (2) Distill under reduced pressure after completion of the reaction, remove the front cut, and obtain 311g of acrylic acid trichloroacetic anhydride.
[0047] (3) Add 311g of acid anhydride into the solvent, lower the temperature to 0°C, add 186g of morpholine dropwise, and add 158.5g of N-methylmorpholine, keep the reaction temperature at 0°C, and drop the reaction for 4 hours to obtain the crude acryloyl Morpholine.
[0048] (4) Filtrate the crude acryloylmorpholine, add a polymerization inhibitor to distill under reduced pressure, collect fractions at 140-160°C, and obtain 181 g of the product, which is the final product acryloylmorpholine. The polymerization inhibitor is methyl ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com