Iridium complex containing 4-phenylpyrimidine structure and application of iridium complex
A technology of phenylpyrimidine and iridium complexes is applied in optoelectronic materials and application fields to achieve the effects of high-efficiency white electroluminescence performance, reduction of molecular energy gap, and improvement of device performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
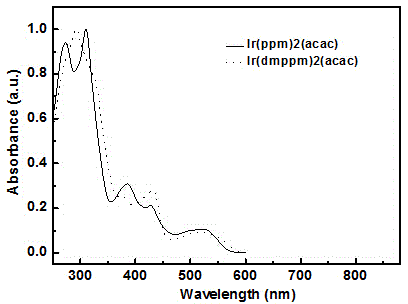
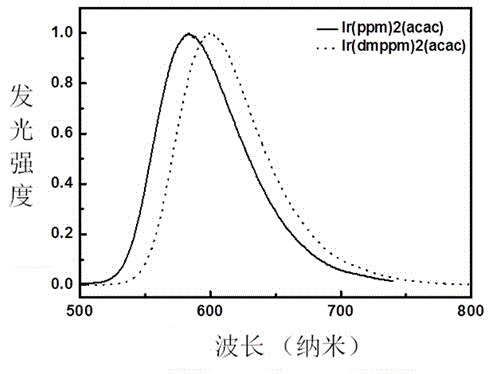
Image
Examples
Embodiment 1
[0046] Step 1: Add 4.00 g of 2,4-dichloropyrimidine and 6.87 g of 3,4,5-trimethyl-phenylboronic acid into a 50 ml flask, add the catalyst Pd(PPh 3 ) 4 650 mg, 30 ml of tetrahydrofuran, 2M K 2 CO 3 The solution was 10 ml, refluxed under argon atmosphere for 24 hours. After cooling, it was extracted with dichloromethane. The organic layer was dried over anhydrous sodium sulfate and spin-dried. Then, it was passed through the column with dichloromethane / petroleum ether=1:3 (volume ratio) and swirled. After drying, 5.1 g of 2,4-diphenylpyrimidine was obtained, and the yield was 82.0%.
[0047] Step 2: Add 4.00 g of 2,4-diphenylpyrimidine and 2.57 g of iridium trichloride hydrate into a 50 ml flask, add 30 ml of diethylene glycol, 5.0 ml of water, and heat to 120°C under argon atmosphere to reflux 24 After hours, the solid obtained after cooling was washed with water, methanol, diethyl ether and n-hexane in sequence to obtain the dichloro bridge substitution complex.
[0048] Step 3: V...
Embodiment 2
[0050] Step 1: Add 4.00 g of 2,4-dichloropyrimidine and 7.67 g of 4-methylphenylboronic acid into a 50 ml flask, add the catalyst Pd(PPh 3 ) 4 650 mg, 30 ml of tetrahydrofuran, 2M K 2 CO 3 The solution was 10 ml, refluxed under argon atmosphere for 24 hours. After cooling, it was extracted with dichloromethane. The organic layer was dried over anhydrous sodium sulfate and spin-dried. Then, it was passed through the column with dichloromethane / petroleum ether=1:3 (volume ratio) and swirled. 6.0 g of 2,4-bis-(4-methylphenyl)pyrimidine was obtained by drying, and the yield was 86.0%.
[0051] Step 2: Add 4.00 g of 2,4-bis-(4-methylphenyl) pyrimidine and 2.29 g of iridium trichloride hydrate into a 50 ml flask, add 30 ml of diethylene glycol, 5.0 ml of water, and heat to Reflux under argon atmosphere at 120°C for 24 hours. After cooling, the solid obtained was washed with water, methanol, diethyl ether and n-hexane in sequence to obtain the dichloro bridge substitution complex.
[00...
Embodiment 3
[0054] Step 1: Add 4.00 g of 2,4-dichloropyrimidine and 8.03 g of 3,5-dimethylphenylboronic acid into a 50 ml flask, add the catalyst Pd(PPh 3 ) 4 650 mg, 30 ml of tetrahydrofuran, 2M K 2 CO 3 The solution was 10 ml, refluxed under argon atmosphere for 24 hours. After cooling, it was extracted with dichloromethane. The organic layer was dried over anhydrous sodium sulfate and spin-dried. Then, it was passed through the column with dichloromethane / petroleum ether=1:3 (volume ratio) and swirled. 6.8 g of 2,4-bis(3,5-dimethyl-phenyl)pyrimidine was obtained by drying, and the yield was 88.0%.
[0055] Step 2: Add 4.00g of 2,4-bis(3,5-dimethylphenyl)pyrimidine and 2.57g of iridium trichloride hydrate into a 50ml flask, add 30ml of diethylene glycol and 5.0ml of water, Heat to 120°C under argon atmosphere and reflux for 24 hours. After cooling, the solid obtained is washed with water, methanol, diethyl ether and n-hexane in sequence to obtain the dichloro bridge substitution complex.
...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com