Fluorescence probe for detecting glutathione as well as preparation method and use method of fluorescence probe
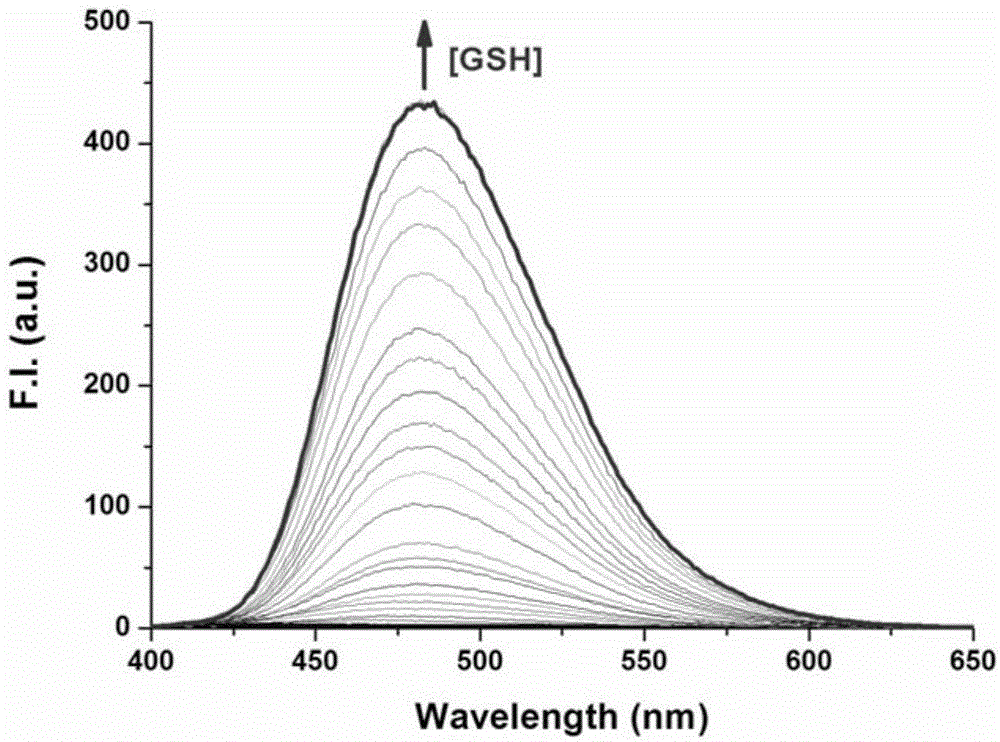
A glutathione and fluorescent probe technology, applied in the direction of fluorescence/phosphorescence, chemical instruments and methods, luminescent materials, etc., can solve problems such as difficult to achieve specific detection of glutathione, and achieve easy promotion and application, excellent Responsive sensitivity, responsive effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
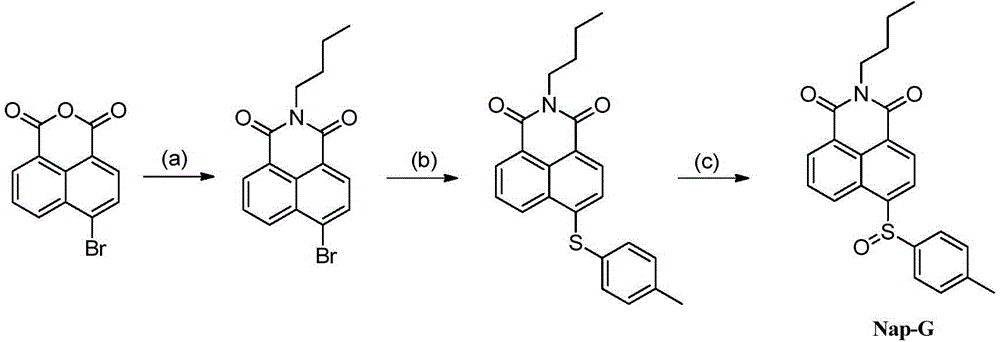
[0057] Such as figure 1 Shown, the preparation of embodiment 1, fluorescent probe Nap-G
[0058] Step a): Under an inert atmosphere, add 5.00g of 4-bromo-1,8-naphthalene dicarboxylic anhydride to 100mL of absolute ethanol, then inject 2.5mL of n-butylamine, and reflux at a temperature of 50°C for 6 Hour. After the reaction was complete, needle-like crystals precipitated after standing overnight, filtered and washed three times with cold ethanol to obtain 4.20 g of intermediate N-n-butyl-4-bromo-1,8-naphthalimide (80% yield).
[0059] Step b): Under an inert atmosphere, add 1.00g of N-n-butyl-4-bromo-1,8-naphthalimide and 1.87g of p-methylthiophenol into a 50mL three-necked flask, inject 20mL of Alcohol monomethyl ether and 2.1 mL of triethylamine were reacted under reflux for 5 hours at a temperature of 0°C. After the reaction is complete, the reaction solution is poured into 200mL of ice water, a large amount of solids precipitate out, filtered, washed, and dried in vacuo ...
Embodiment 2
[0063] Embodiment 2, the preparation of fluorescent probe Nap-G
[0064] Step a): Under an inert atmosphere, add 5.00g of 4-bromo-1,8-naphthalic anhydride to 100mL of anhydrous methanol, then inject 5.0mL of n-butylamine, and reflux at a temperature of 80°C for 1 Hour. After the reaction was complete, needle-like crystals precipitated after standing overnight, filtered and washed three times with cold ethanol to obtain 4.40 g of intermediate N-n-butyl-4-bromo-1,8-naphthalimide (yield 84%).
[0065] Step b): Under an inert atmosphere, add 1.00g of N-butyl-4-bromo-1,8-naphthalimide and 3.94g of p-methylthiophenol into a 50mL three-necked flask, inject 20mL of butanol and 2.5 mL of diisopropylethylamine was refluxed at 100°C for 1 hour. After the reaction is complete, the reaction solution is poured into 200mL of ice water, a large amount of solids precipitate out, filtered, washed, and dried in vacuo to obtain N-n-butyl-4-(p-methylphenylsulfanyl)-1,8-naphthalene Amine 1.0 g (...
Embodiment 3
[0069] Embodiment 3, the preparation of fluorescent probe Nap-G
[0070] Step a): Under an inert atmosphere, add 5.00g of 4-bromo-1,8-naphthalic anhydride to 100mL of anhydrous n-propanol, then inject 7.5mL of n-butylamine, and reflux at a temperature of 120°C React for 5 hours. After the reaction was complete, needle-like crystals precipitated after standing overnight, filtered and washed three times with cold ethanol to obtain 4.60 g of intermediate N-n-butyl-4-bromo-1,8-naphthoimide (yield 87%).
[0071] Step b): Under an inert atmosphere, add 1.00g of N-n-butyl-4-bromo-1,8-naphthalimide and 0.38g of p-methylthiophenol into a 50mL three-necked flask, inject 20mL of diethyl Glycol monomethyl ether and 2.9 g of potassium carbonate were refluxed for 48 hours at a temperature of 140°C. After the reaction is complete, the reaction solution is poured into 200mL of ice water, a large amount of solids precipitate out, filtered, washed, and dried in vacuo to obtain N-n-butyl-4-(p-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com