Genetic engineering recombinant high-purity anti-mycotoxin monoclonal antibody for inhibiting and killing mycotoxin as well as composite monoclonal antibody preparation method and combined preparation thereof
A mycotoxin and genetic engineering technology, applied in antifungal agents, antibodies, antifungal/algae/lichen immunoglobulins, etc., can solve the problems of feed additives without mycotoxins, unstable treatment effects, and mycotoxin hazards. Achieve natural safety toxic side effects and residues, improve biological activity and consistency, and ensure food safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0051] The preparation method of the genetically engineered recombinant high-purity anti-mycotoxin compound monoclonal antibody for inhibiting mycotoxins used in the present invention comprises the following steps:
[0052] 1. Screen representative mycotoxins
[0053] Mycotoxins mainly refer to the toxic metabolites produced by molds in the process of contaminating substrates. Due to the diversity of mold species, their toxic metabolites are also diverse. There are more than 200 known mycotoxins. Among them, the main mycotoxins that are most harmful to animals and humans are: aflatoxin (mainly AFB1), zearalenone, deoxynivalenol (deoxynivalenol), ochratoxin A, citrinin, and fumonisins B1, penicillin acid. Their characteristics and hazards are as follows:
[0054] (1) Mycotoxins: Aflatoxins are the most harmful and common mycotoxins produced by Aspergillus flavus and Aspergillus parasiticus through the polyketide pathway, and their toxicity is much higher than that of cyanide...
experiment example 1
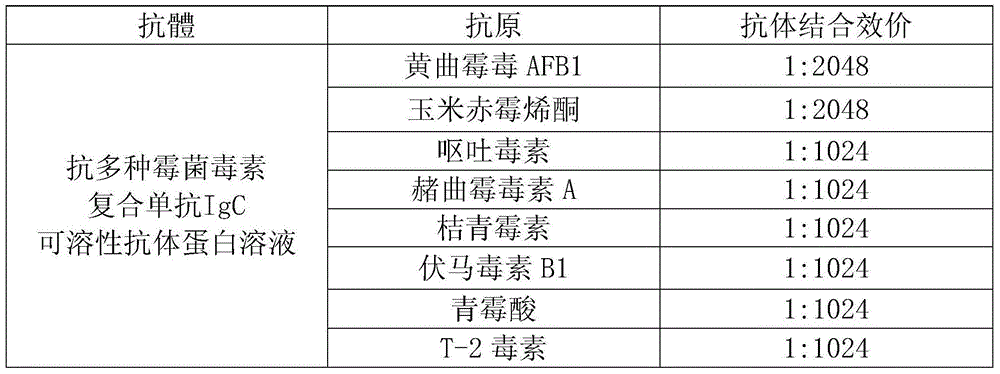
[0156] Antibody binding titer detection of multiple mycotoxin compound monoclonal antibody IgC to common mycotoxins
[0157] Detection of anti-multiple mycotoxin compound monoclonal antibody IgC against aflatoxin AFB1, zearalenone, deoxynivalenol, ochratoxin A, citrinin, fumonisin B1, penicillin, T Antibody binding titers of 8 antigens including -2 toxins:
[0158] Eight kinds of mycotoxins including aflatoxin AFB1, zearalenone, deoxynivalenol, ochratoxin A, citrinin, fumonisin B1, penicillic acid and T-2 toxin were used as the Detect the antigen, and use the "ELISA" method (enzyme-linked immunoassay) to detect the antibody titer of the prepared anti-multiple mycotoxin complex monoclonal antibody IgC soluble antibody protein solution, and the results are shown in the table below.
[0159]
[0160] Note: The concentration of the anti-multiple mycotoxin complex monoclonal antibody IgC soluble antibody protein solution in the test sample is 1mg / mL.
[0161] It can be seen fr...
experiment example 2
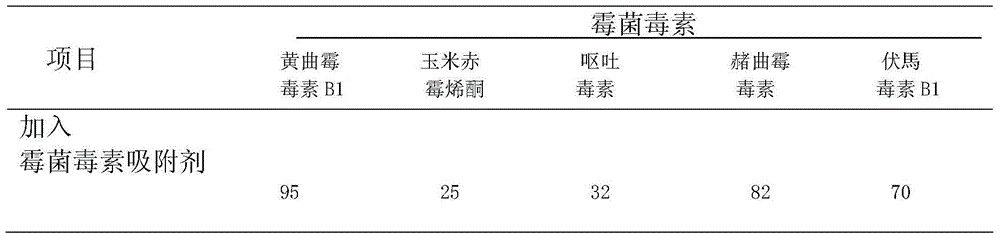
[0163] Anti-Multiple Mycotoxin Compound Monoclonal Antibody IgC Degradation Effect of Common Mycotoxins
[0164] 1. Materials
[0165] (1) Anti-multiple mycotoxin compound monoclonal antibody IgC soluble protein dry powder
[0166] (2) Standard mycotoxin working solution (pH 5.0)
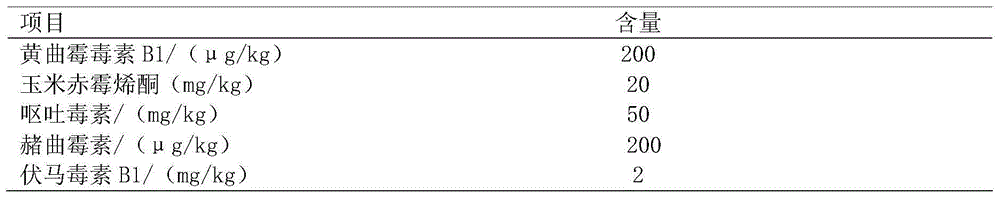
[0167] Aflatoxin AFB1, zearalenone, deoxynivalenol, ochratoxin A, citrinin, fumonisin B1, penicillic acid, T-2 toxin and other 8 mycotoxin standard products were purchased from Singapore Pribo Laboratory . Prepare mycotoxin working solution with pH 5.0 phosphate buffer. The content of mycotoxins in the working solution is set according to the national limit standard of mycotoxins in feed and the actual production situation.
[0168] (3) Instruments and kits:
[0169] THZ-82 constant temperature water bath shaker; Bio-Rad iMarkTM microplate reader; SK-1 fast mixer; Kubota 2420 high-speed centrifuge; aflatoxin B1, zearalenone, deoxynivalenol, ochratoxin A , citrinin, fumonisin B1, penicillin, T-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com