Method for preparing 4-methyl-4-trichloromethyl-2, 5-cyclohexadiene-1-ketone
A technology of trichloromethyl and cyclohexadiene, applied in the field of chemical intermediate preparation, can solve problems such as unfavorable industrial production, environmental protection and safety, harsh production conditions, etc., and achieves convenient industrial production, no irritating odor, and reduced reaction cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] In this embodiment, a preparation method of 4-methyl-4-trichloromethyl-2,5-cyclohexadien-1-one comprises the following steps:
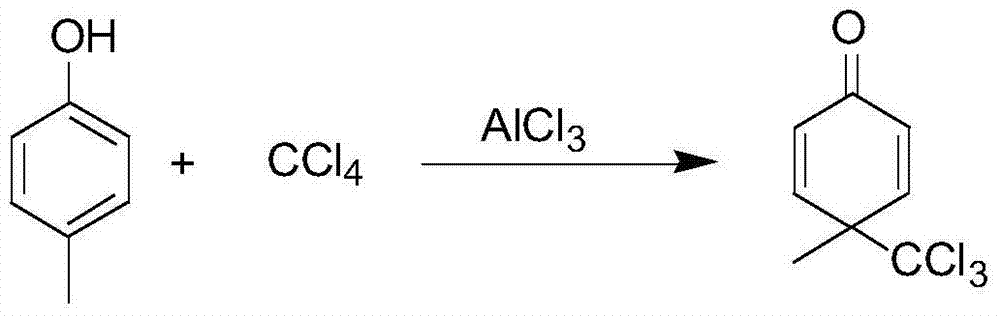
[0043] S1. Weigh 28g of aluminum trichloride and add it to 116g of dichloromethane, stir in the reaction bottle and cool down to 5°C; weigh 15g of p-cresol and dissolve it in 50g of dichloromethane, and place it in a constant pressure dropping funnel. Then slowly add it to the dichloromethane reaction system of aluminum trichloride. During the dropwise addition, the reaction temperature is 15°C. hour, the point plate reaction is complete;
[0044] Among them, p-cresol and carbon tetrachloride are used as raw materials to prepare 4-methyl-4-trichloromethyl-2,5-cyclohexadiene-1- Ketones, the reaction equation is as follows:
[0045]
[0046] S2. Cool the mixed solution prepared in S1 to room temperature, then slowly add it into 150 g of ice water, and stir for 15 minutes;
[0047] S3, liquid separation, wherein the liquid separation temperatu...
Embodiment 2
[0051] In this embodiment, a preparation method of 4-methyl-4-trichloromethyl-2,5-cyclohexadien-1-one comprises the following steps:
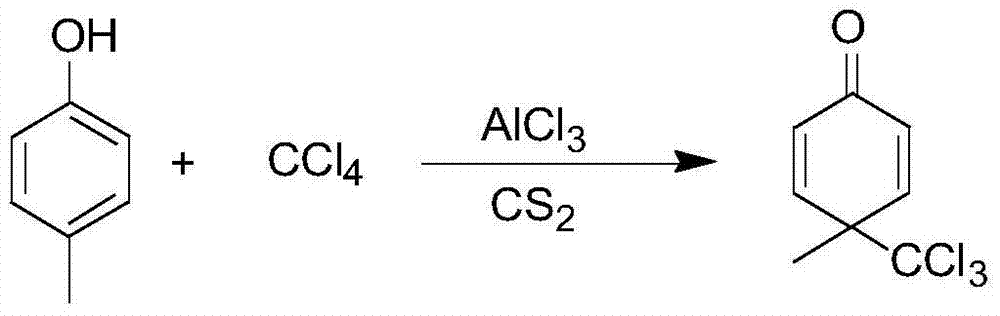
[0052] S1. Weigh 70g of aluminum trichloride and add it to 120g of dichloromethane, stir in the reaction bottle and cool down to 10°C; weigh 37g of p-cresol and dissolve it in 160g of dichloromethane, and place it in a constant pressure dropping funnel. Then slowly add it to the dichloromethane reaction system of aluminum trichloride. During the dropwise addition, the reaction temperature is 5°C. Less than 2 hours, the plate reaction is complete;
[0053] Among them, p-cresol and carbon tetrachloride are used as raw materials to prepare 4-methyl-4-trichloromethyl-2,5-cyclohexadiene-1- Ketones, the reaction equation is as follows:
[0054]
[0055] S2. Cool the mixed solution prepared in S1 to room temperature, then slowly add it to 320 g of ice water, and stir for 45 minutes;
[0056] S3, liquid separation, wherein the liquid separation t...
Embodiment 3
[0060] In this embodiment, a preparation method of 4-methyl-4-trichloromethyl-2,5-cyclohexadien-1-one comprises the following steps:
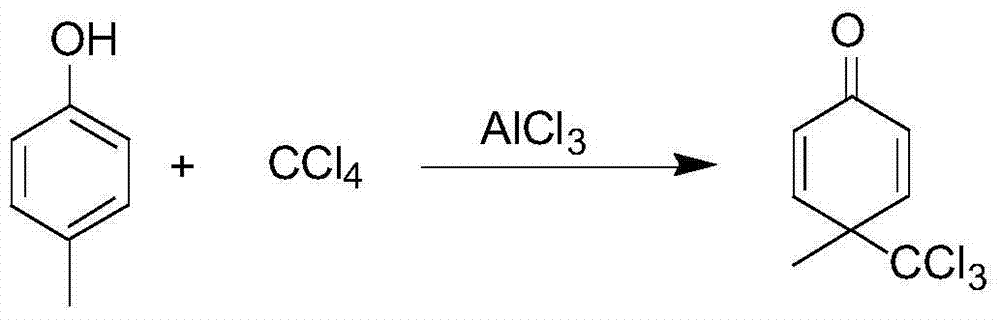
[0061] S1. Weigh 32g of aluminum trichloride and add it to 134g of dichloromethane, stir in the reaction bottle and cool down to 7°C; weigh 20g of p-cresol and dissolve it in 66g of dichloromethane, and place it in a constant pressure dropping funnel. Then slowly join in the dichloromethane reaction system of aluminum trichloride, when dropwise, reaction temperature is 12 ℃, after dropwise is finished, continue to add carbon tetrachloride 28g, raise temperature to 38 ℃, continue to stir for 3 hour, the point plate reaction is complete;
[0062] Among them, p-cresol and carbon tetrachloride are used as raw materials to prepare 4-methyl-4-trichloromethyl-2,5-cyclohexadiene-1- Ketones, the reaction equation is as follows:
[0063]
[0064] S2. Cool the mixed solution prepared in S1 to room temperature, then slowly add it to 200 g of ice water...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com