Method for preparing uniform carbon-coated vanadium phosphate sodium material
A carbon-coated sodium vanadium phosphate, carbon-coated technology, applied in chemical instruments and methods, phosphorus compounds, inorganic chemistry, etc., can solve the problems of uneven carbon layer coating, large particle size distribution range, etc., and achieve inhibition of agglomeration phenomenon, coating uniformity, and the effect of improving conductivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] 4mmolV 2 o 5 , 12 mmol NH 4 h 2 PO 4 , 6 mmol Na 2 CO 3 Dissolve in 70ml of distilled water, then add 6mmol of glucose and stir evenly, transfer the above solution to the reaction kettle, heat it in water at 180°C for 12h, cool it at room temperature, open the kettle, ultrasonicate the obtained suspension for 90min, and then heat it at 70°C The water was evaporated with magnetic stirring in a water bath to obtain a sol, and the sol was dried in an oven at 60° C. for 12 hours to obtain a gel. The above gel was fully ground and pre-calcined in argon at 350 °C for 4 h; the pre-fired sample was fully ground again and calcined in argon at 650 °C for 8 h to obtain the product. The prepared Na 3 V 2 (PO 4 ) 3 The scanning electron microscope photograph of the material is as figure 1 As shown, it shows that the prepared products have uniform size.
Embodiment 2
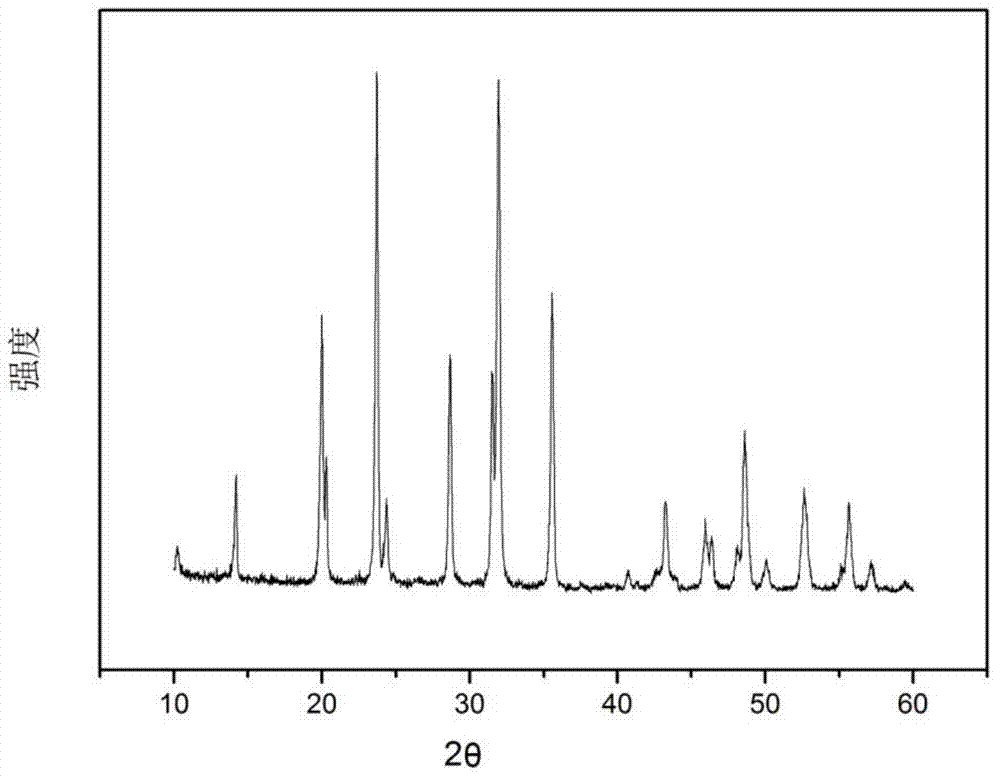
[0026] 4mmolV 2 o 5 , 12 mmol NH 4 h 2 PO 4 , 6 mmol Na 2 CO 3 Dissolve in 70ml of distilled water, then add 3mmol of glucose and stir evenly, transfer the above solution to a reaction kettle, heat it in water at 180°C for 40h, cool it at room temperature and open the kettle, ultrasonicate the obtained suspension for 90min, and then heat it at 95°C The water was evaporated by magnetic stirring in a water bath to obtain a sol, and the sol was dried in an oven at 80° C. for 6 hours to obtain a gel. Grind the above gel thoroughly and pre-calcine in argon at 350°C for 4 hours; grind the pre-fired sample fully again, and calcinate in argon at 800°C for 6 hours to obtain the product. The prepared Na 3 V 2 (PO 4 ) 3 The X-ray diffraction pattern of the material is as figure 2 As shown, the prepared product has a pure composition.
Embodiment 3
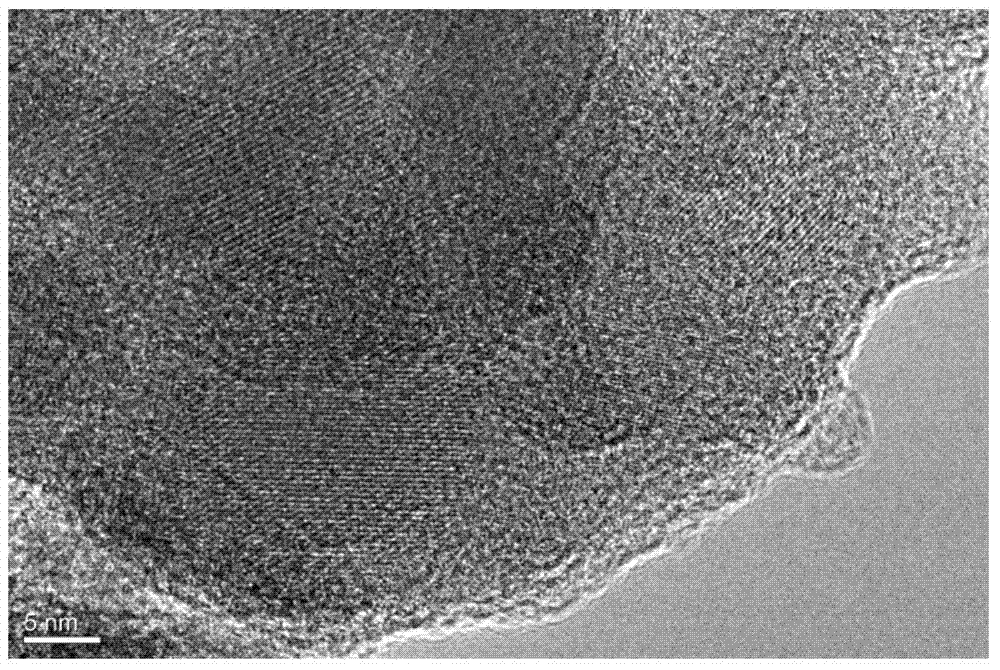
[0028] 4mmolV 2 o 5 , 12 mmol NH 4 h 2 PO 4 , 6 mmol Na 2 CO 3 Dissolve in 70ml of distilled water, then add 4.5mmol of glucose and stir evenly, transfer the above solution to a reaction kettle, heat it in water at 180°C for 24h, cool it at room temperature and open the kettle, ultrasonicate the obtained suspension for 90min, and then in 80 The water was evaporated by magnetic stirring in a water bath at °C to obtain a sol, and the sol was dried in an oven at 70 °C for 9 hours to obtain a gel. The above gel was thoroughly ground and pre-calcined in argon at 350 °C for 4 h; the pre-fired sample was fully ground again, and calcined in argon at 750 °C for 7 h to obtain the product. The prepared Na 3 V 2 (PO 4 ) 3 The transmission electron microscope pictures of the material are as image 3 As shown, it shows that the surface of the prepared product is coated with a uniform carbon layer.
[0029] It can also be clearly seen from the accompanying drawings of the above ex...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com