Glucose sensitive controlled release microballoon composition and preparation method thereof
A composition and microsphere technology, which can be applied in the directions of drug combination, medical preparations with non-active ingredients, non-active ingredients of polymer compounds, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
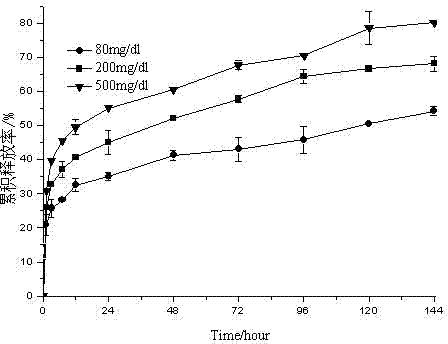
Embodiment 1
[0041] Weigh 250 mg of ketal polymer material with a molecular weight of 4200, 6 mg of glucose oxidase, and 6 mg of catalase, add 2 mL of dichloromethane to dissolve, and form a homogeneous suspension as the oil phase; 12 mg of bovine insulin is dissolved in 50 μL of DMSO As a drug solution; put the oil phase in a homogenizer (IKA T25, Staufenim Breisgau, Germany) at 7500r min -1 Under homogeneous conditions, the drug solution was dropped into the oil phase, and the anti-solvent effect caused the precipitation of insulin to form a uniform S / O suspension, which continued to be homogenized for 2 minutes; -1Drop into 66mL PVA aqueous solution with a concentration of 10mg / ml under magnetic stirring conditions to form S / O / W double emulsion; stir at low speed, volatilize the organic solvent for 8 hours, and solidify the microspheres; pass through a 1200-mesh sieve and deionize at 0°C The microspheres were washed with water for 3 times, transferred to a petri dish, and freeze-dri...
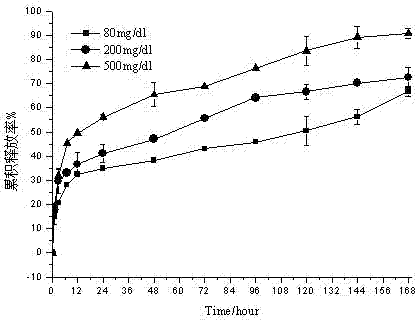
Embodiment 2
[0043] Weigh 250 mg of ketal polymer material with a weight average molecular mass of 4200, 24 mg of glucose oxidase, and 24 mg of catalase, add 2 mL of dichloromethane to dissolve, and form a homogeneous suspension as the oil phase; 12 mg of bovine insulin is dissolved in 50 μL of DMSO was used as a drug solution; the oil phase was placed in a homogenizer (IKA T25, Staufenim Breisgau, Germany), at 25000r min -1 The drug solution was dropped into the oil phase under homogeneous conditions, and the anti-solvent effect caused insulin to precipitate to form a uniform S / O suspension, which lasted for 2 minutes; the above solution was dropped into the oil phase under the condition of 1500r·min-1 magnetic stirring Form S / O / W double emulsion in 50mL of PVA aqueous solution with a concentration of 10mg / ml; stir in an ice-water bath at low speed for 8h, volatilize the organic solvent, and solidify the microspheres; pass through a 1200-mesh sieve, and wash the microspheres with deio...
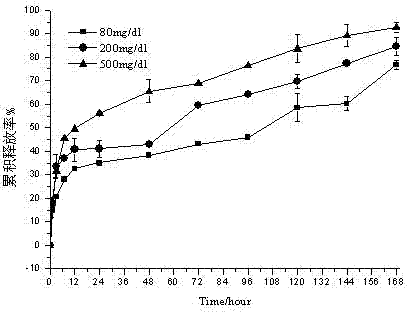
Embodiment 3
[0045] Weigh 100 mg of ketal polymer material with a weight average molecular weight of 2200, 6 mg of glucose oxidase, and 6 mg of catalase, add 2 mL of dichloromethane to dissolve, and form a homogeneous suspension as the oil phase; 12 mg of bovine insulin is dissolved in 50 μL of In DMSO as a drug solution; the oil phase is placed in a homogenizer, at 13000r min -1 The drug solution was dropped into the oil phase under homogeneous conditions, and the anti-solvent effect caused insulin to precipitate to form a uniform S / O suspension, which lasted for 2 minutes; the above solution was dropped into the oil phase under the condition of 1500r·min-1 magnetic stirring Form S / O / W double emulsion in 118mL PVA aqueous solution with a concentration of 10mg / ml; stir in an ice-water bath at low speed for 8h, volatilize the organic solvent, and solidify the microspheres; pass through a 1200-mesh sieve, and wash the microspheres with deionized water at 0°C 3 times, transferred to a petri d...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| encapsulation rate | aaaaa | aaaaa |
| encapsulation rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com