Preparation method of titanium dioxide and doped body of titanium dioxide
A technology of titanium dioxide and titanium salt, which is applied in the field of preparation of titanium dioxide and its doped body, can solve the problems that it cannot be used as a photocatalyst alone and is easy to decompose when exposed to light, and achieves the effects of uniform size, simple preparation method, and cheap and easy-to-obtain raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
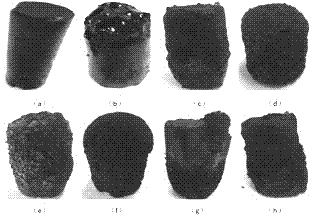
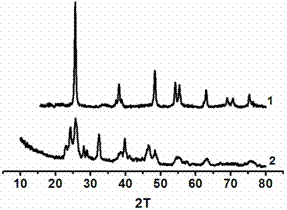
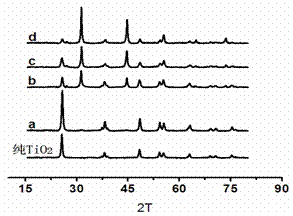
[0023] Dissolve 10g of glucose and 6g of polyvinylpyrrolidone (K30) in 60mL of water, then add 10mL of acetic acid, 4.3g of n-tetrabutyl titanate, 0.298g of potassium iodide and 0.315g of silver nitrate, wherein the molar weight of potassium iodide and silver nitrate is titanate 15% of n-tetrabutyl ester, the mixed solution was transferred to a closed reaction kettle, and reacted at 200°C for 5 hours, and a columnar gel was obtained after the reaction. figure 1 (a), after the gel was dried, it was calcined at 500 °C for 3 hours to obtain a silver iodide-doped titanium dioxide photocatalyst, and its powder diffraction pattern is shown in figure 2 , it can be seen from the figure that the synthesized titanium dioxide powder is anatase phase (PDF No.21-1272), and there are obvious peaks of silver iodide (PDF No.09-0374); Degradation effect see Figure 4 (2).
Embodiment 2
[0025] Dissolve 5.5g sucrose and 0.5g polyacrylamide (Mw: 2000000~14000000) in 20mL water, then add 4.3g n-tetrabutyl titanate, 0.147g potassium bromide and 0.21g silver nitrate, among which potassium bromide and nitric acid The molar weight of silver is 10% of that of n-butyl titanate. Transfer the mixed solution to a closed reaction kettle and react at 200°C for 5 hours. After the reaction, a cylindrical gel is obtained. See figure 1 (b), After the gel was dried, it was calcined at 500 °C for 3 hours to obtain the silver bromide-doped titania photocatalyst.
Embodiment 3
[0027] Dissolve 5g of lactose and 0.6g of polyacrylamide (Mw: 2000000~14000000) in 15mL of water, add 10mL of acetic acid, and then add 2.37g of titanium tetrachloride, 0.019g of potassium iodide and 0.02g of silver nitrate, of which, potassium iodide and silver nitrate The molar weight is 1% of titanium tetrachloride. Transfer the mixed solution to a closed reaction kettle and react at 200°C for 5 hours. After the reaction, a cylindrical gel is obtained. After the gel is dried, it is calcined at 500°C for 3 hours to obtain Silver iodide doped titanium dioxide photocatalyst.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com