Docetaxel nano polymer micelle freeze-drying preparation and preparation method thereof
A nanopolymer, docetaxel technology, applied in the field of medicine, can solve the problems of drug leakage, unsuitable for large-scale production, unable to be further promoted and truly applied, and achieve the effect of improving stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
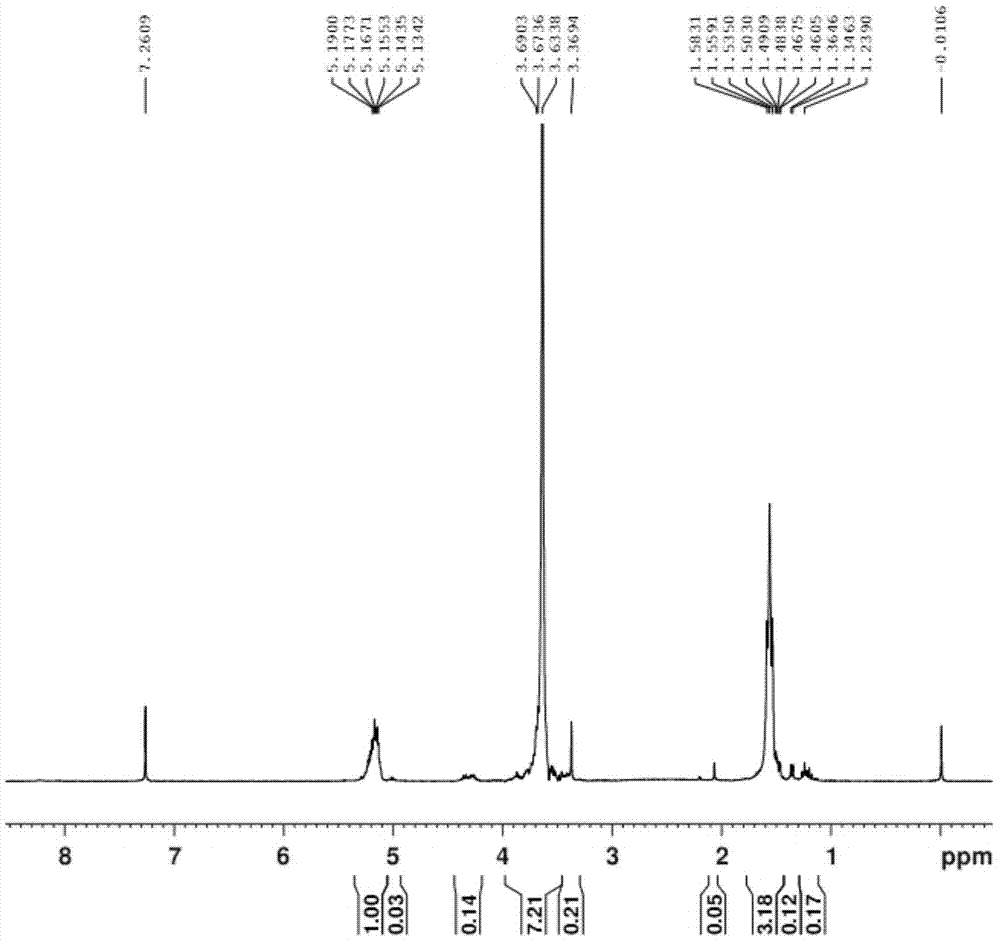
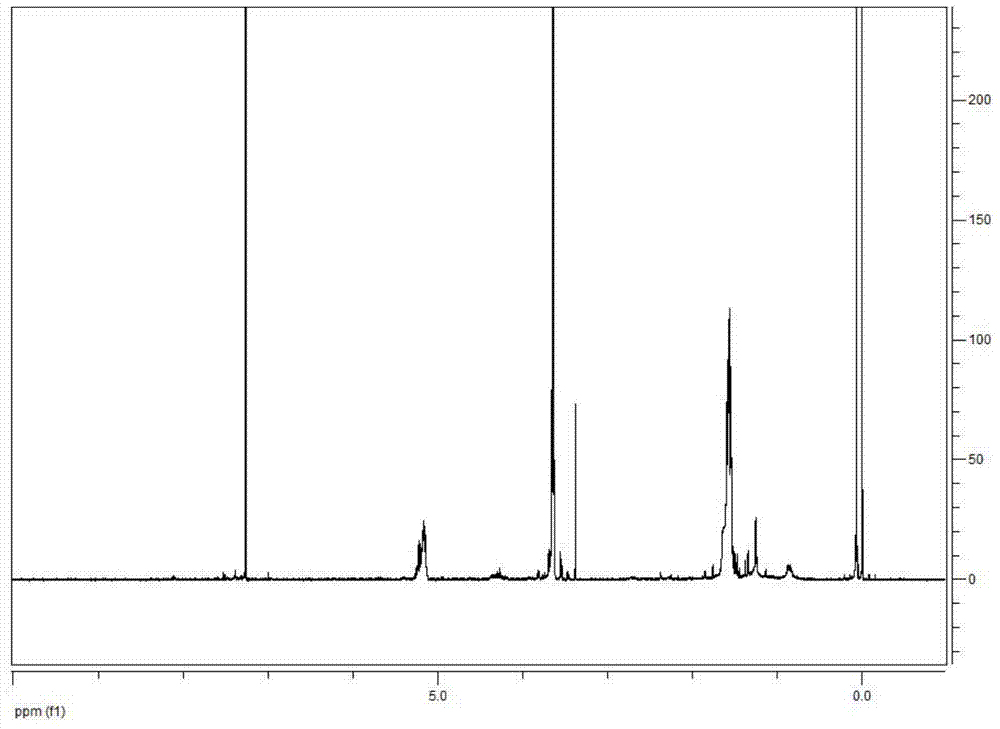
[0032] Example 1 Preparation of polyethylene glycol monomethyl ether-polylactic acid block polymer.
[0033] (1) Weigh 51.07g of D,L-lactide and 50.57g of polyethylene glycol monomethyl ether 2000 for later use, dry polyethylene glycol monomethyl ether 2000 in vacuum at 100°C for 7 hours, replace with nitrogen, add D, For L-lactide, put in 0.2g of catalyst stannous octoate, vacuumize to a vacuum degree of 0.096Mpa, seal it, and keep the reaction temperature at 100°C. After the D and L-lactide are all melted, replace with nitrogen three times, and then pump Vacuum to ensure the negative pressure in the reactor, airtight, heat up to 140 ° C, react for 12 hours, the reaction is complete, and a light yellow clear viscous liquid is obtained.
[0034](2) Add dichloromethane to the light yellow clear viscous liquid obtained in step (1), add 25ml of dichloromethane, stir for 30min; then add 510ml of anhydrous glacial ether, stir for 30min; then statically Set aside for 12 hours, vacu...
Embodiment 2
[0035] Example 2 Preparation of polyethylene glycol monomethyl ether-polylactic acid block polymer.
[0036] (1) Weigh 48.77g of D,L-lactide and 51.27g of polyethylene glycol monomethyl ether 2000 for later use, dry polyethylene glycol monomethyl ether 2000 at 120°C for 5 hours in vacuum, replace with nitrogen, and put into D,L -Lactide, then put in 0.048g catalyst stannous octoate, vacuumize to a vacuum degree of 0.095Mpa, maintain the reaction temperature at 120°C, after the D,L-lactide is completely melted, replace with nitrogen for 3 times, and then pump Vacuum to ensure the negative pressure in the reactor, nitrogen protection, then raise the temperature to 140 ° C, react for 14 hours, the reaction is complete, and a light yellow clear liquid is obtained.
[0037] (2) Add 29ml of dichloromethane to the above light yellow clear liquid to dissolve, stir to dissolve; then add 586ml of ice anhydrous ether, stir for 30min; stand at 5°C for 12h, then vacuum dry by suction filtr...
Embodiment 3
[0038] Example 3 Preparation of polyethylene glycol monomethyl ether-polylactic acid block polymer.
[0039] (1) Weigh 47.53g of D,L-lactide and 52.17g of polyethylene glycol monomethyl ether 2000 for later use, dry polyethylene glycol monomethyl ether 2000 at 130°C for 7 hours in vacuum, replace with nitrogen, and put in 0.3g of catalyst Stannous octoate, then put in D, L-lactide, evacuate to a vacuum degree of 0.093Mpa, maintain the reaction temperature at 130°C, after the D, L-lactide is completely melted, replace with nitrogen for 3 times, and then evacuate , ensure negative pressure in the reactor, airtight, then raise the temperature to 150° C., react for 6 hours, and the reaction is completed to obtain a light yellow clear liquid.
[0040] (2) Add 45ml of dichloromethane to the light yellow clear liquid in step (1), stir to dissolve; then add 550ml of ice anhydrous diethyl ether, stir for 30min; stand at 0°C for 12h, then vacuum dry by suction filtration. Purification ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com