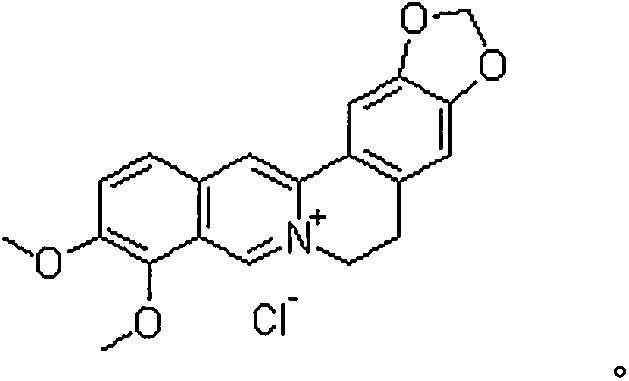
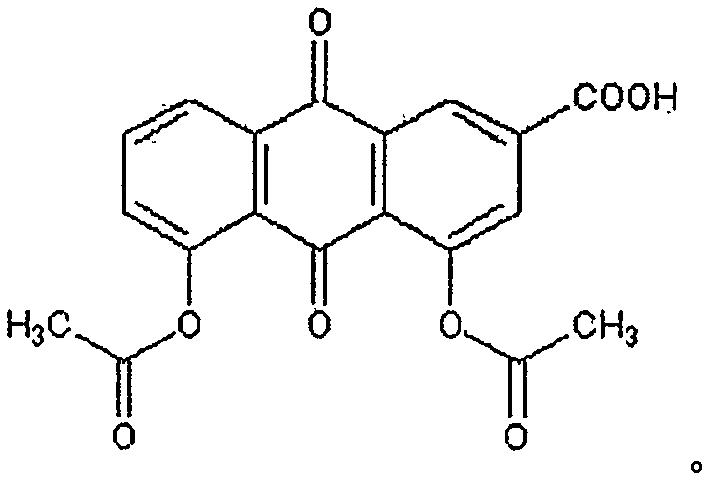
Oral pharmaceutical composition of diacerein and berberine
A technology of diacetyl rhein and a composition is applied in the field of compound preparation and preparation of berberine and diacetyl rhein, can solve problems such as lack of therapeutic drugs, and achieves simple preparation method, good stability and simple product prescription. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] The prescription of ordinary tablets (1000 tablets) is shown in Table 1:
[0047] Table 1 Prescription of ordinary tablets (1000 tablets) (the following weight unit is g)
[0048]
[0049]
[0050] Preparation Process:
[0051] (1) Premixing active pharmaceutical ingredients, fillers and binders or ethanol solution and wet granulation;
[0052] (2) drying and sizing the granules prepared by wet granulation to obtain dry granules;
[0053] (3) blending dry granules with disintegrating agent and lubricant to obtain blended granules;
[0054] (4) compressing the mixed granules into tablets to obtain plain tablets;
[0055] (5) Carry out common film coating to plain tablet coating material;
[0056] Wet granulation can use wet granulator, parameters: 1 stirring, 2 shearing, a total of 4min ~ 8min.
[0057] Granules are dried using a box-type drying oven or a fluidized granulation dryer at a drying temperature of 50°C to 70°C.
[0058] The final mixing adopts th...
Embodiment 2
[0079] The prescription of capsules (1000) is shown in Table 8:
[0080] The prescription of table 8 capsule (1000) (the following weight unit is g)
[0081]
[0082] Preparation Process:
[0083] (1) Premixing active pharmaceutical ingredients, fillers and binders or ethanol solution and wet granulation;
[0084] (2) drying and sizing the granules prepared by wet granulation to obtain dry granules;
[0085] (3) blending dry granules with lubricant to obtain blended granules;
[0086] (4) Capsule filling of the blended granules.
[0087] Wet granulation can use wet granulator, parameters: 1 stirring, 2 shearing, a total of 4min ~ 8min.
[0088] Granules are dried using a box-type drying oven or a fluidized granulation dryer at a drying temperature of 50°C to 70°C.
[0089] The final mixing adopts three-dimensional motion mixer or lifting hopper mixer.
[0090] Capsule filling uses a capsule filling machine.
[0091] The prescription detection conclusion of capsule (...
Embodiment 3
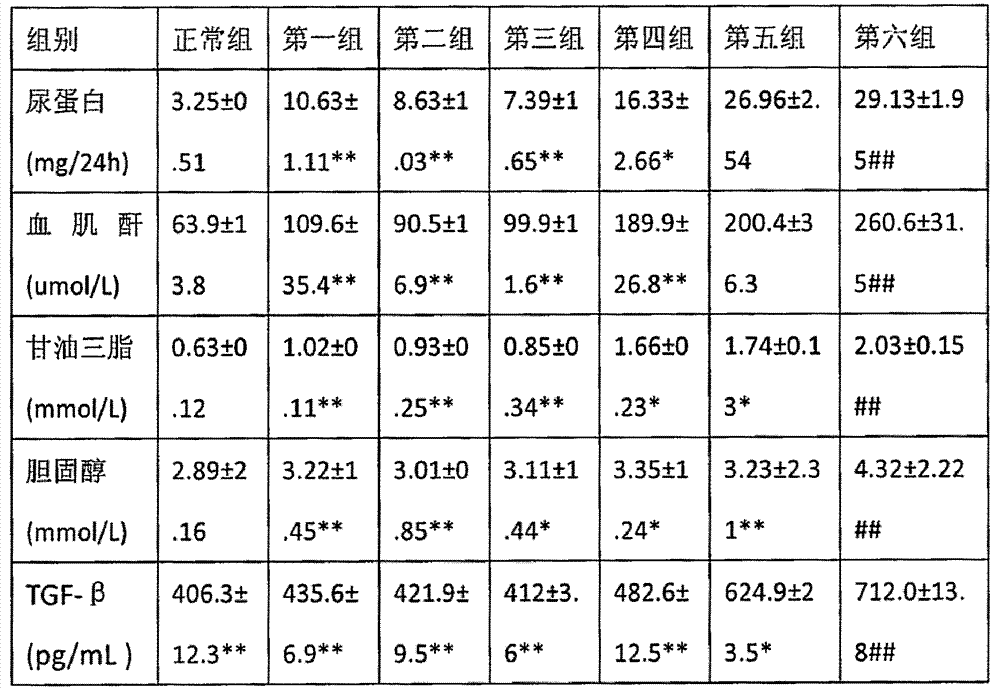
[0106] Streptococcus (STZ) is a kind of widely used medicine that causes rat diabetes model, male Wistar rat body weight 180-220g, single intraperitoneal injection is dissolved in the STZ of pH=4.5 sodium citrate (27.5mg / mL ), calculated as 55mg / kg based on body weight. The control group was injected with the same volume of solvent sodium proxylate (0.2mL / 100g). Three days after the injection, blood was taken from the tail vein to measure blood sugar. Rats with blood glucose greater than or equal to 16.7mmol / L were selected as experimental rats. The screened rats were divided into groups and oral administration was started. Urine was collected for 12 hours (21:00-9:00) every two weeks from the injection of STZ in rat metabolic cages and the urine protein content was determined. After fasting at 21:00 the night before for days 0, 4, 8, and 12, blood was taken from the tail vein at 9:00 the next morning to measure the blood sugar concentration. After the rats in each group we...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com