Fluorescent molecular switch and fluorescent probe thereof, and applications of fluorescent probe
A technology of fluorescent molecules and fluorescent probes, applied in the field of fluorescent probes, can solve the problems of rare organic fluorescent small molecule materials, and achieve the effect of reducing costs and time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
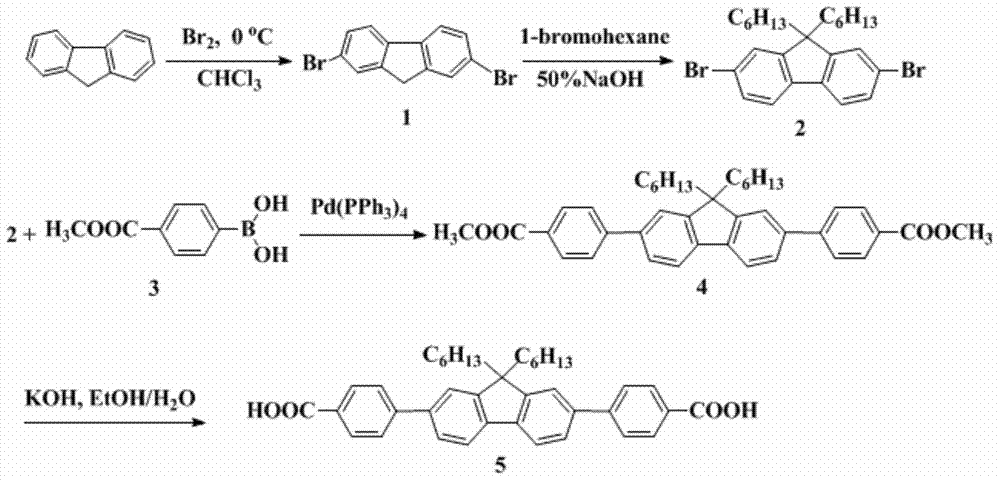
[0049] Synthesis of small molecule derivatives of fluorene (compound 5) (see figure 1 ):
[0050] Add 2 g of fluorene, 20 mL of chloroform, and 10.6 mg of iron powder to a 50 mL round-bottomed flask, cool it to below 0°C in an ice bath, and slowly add a mixture of 4.12 g of liquid bromine and 10 mL of chloroform dropwise. After the dropwise addition, the reaction is continued for 2 hours. Aqueous sodium bisulfite was added to remove excess bromine. The chloroform layer was separated, concentrated, the solid was filtered out, and then purified by recrystallization from chloroform to obtain 2.95 g of 2,7-dibromofluorene (compound 1) as white crystals, yield: 78.5%.
[0051] 1.5 g of 2,7-dibromofluorene, 0.009 g of triethylbenzyl ammonium chloride, and 30 mL of dimethyl sulfoxide were placed in a three-necked flask to form a suspension, and 1.5 mL of 50wt% sodium hydroxide aqueous solution was added dropwise, and the reaction was carried out for half an hour. , 1.59g of 1-bromo...
Embodiment 2
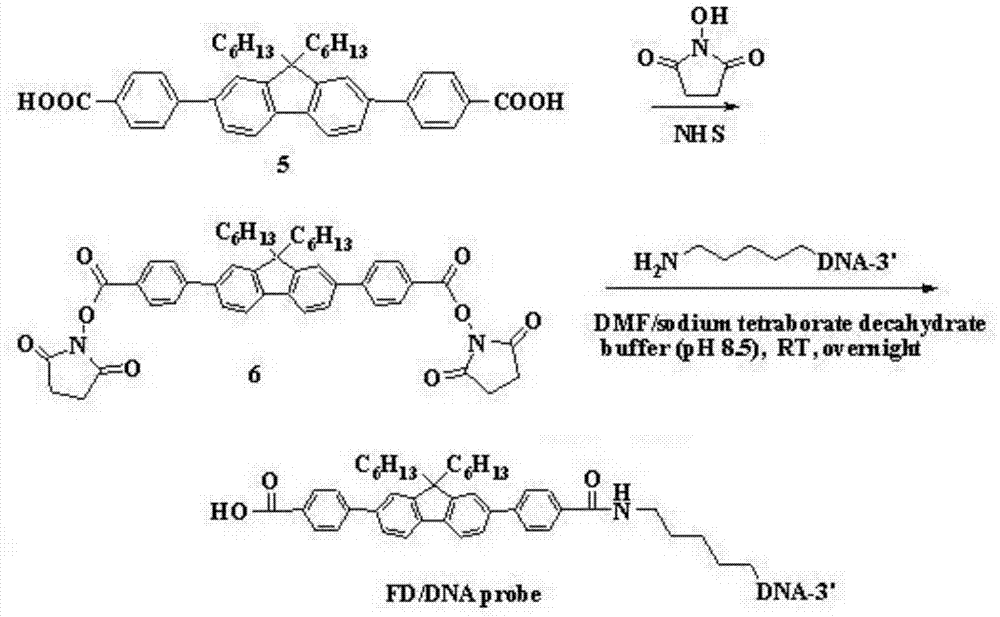
[0057] Design and Synthesis of Small Molecular Derivatives of Fluorene / DNA Probes
[0058] Dissolve compound 5 (100 mg) and N-hydroxysuccinimide (91.25 mg) obtained in Example 1 in 10 mL of anhydrous N,N-dimethylformamide (DMF), and then slowly add 1,3-dimethylformamide (DMF). The solution of cyclohexylcarbodiimide (DCC, 92.75mg) dissolved in 5mL DMF, after adding, react at room temperature for 24h, thin layer chromatography (TLC) detects the end of the reaction, the reaction solution is extracted with dichloromethane, and the solvent is removed under reduced pressure , and column chromatography (petroleum ether: ethyl acetate = 3:1) separated a light yellow solid (compound 6), and its nuclear magnetic detection results were as follows:
[0059] 1 H NMR (600MHz, CDCl 3 ):δ8.17(d,J=8.4Hz,4H),7.75(m,6H),7.59(t,J=7.2Hz,2H),7.54(s,2H),2.87(s,8H),2.00 (m,4H),1.26-0.62(m,22H).
[0060] The above-obtained compound 6 is connected to the 5'-terminal amino group of DNA through the a...
Embodiment 3
[0065] Example 3: Molecular switch construction based on fluorene-based small molecule derivatives (compound 5)
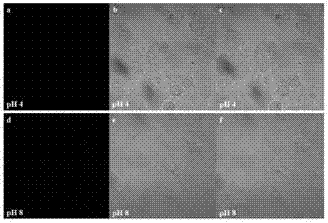
[0066] Compound 5 in Example 1 was dissolved in DMSO to obtain a concentration of 1.7×10 -3 M's mother liquor. The pH value of 2 mL of ultrapure water was adjusted to 1-14 with 5M NaOH and 12M HCl, and a certain amount of mother liquor was added to obtain the working solution. After mixing the samples with a pipette, UV absorption spectroscopy and fluorescence emission spectroscopy were performed. At the same time, compound 6 (dissolved in DMF) in Example 1 was used as a control sample and operated in parallel according to the above method.
[0067] from the attached image 3 It can be seen that the UV absorption peak of compound 5 appears at 350 nm under acidic conditions (pH1-6); and with the increase of pH value, under neutral and basic (pH7-14) conditions, UV absorption peaks appear A blue shift of 10 nm means that the absorption peak is at 340 nm. from th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com