Acid-sensitive polymer carrier for delivering antitumor drugs, and preparation method and application thereof
A polymer carrier and anti-tumor drug technology, applied in the field of acid-sensitive polymer carrier and preparation, can solve the problems of inability to self-assemble to form nanoparticles, lack of stable drug release, low drug loading, etc., to overcome the ineffective clinical treatment effect. Ideal, improve water solubility, reduce toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
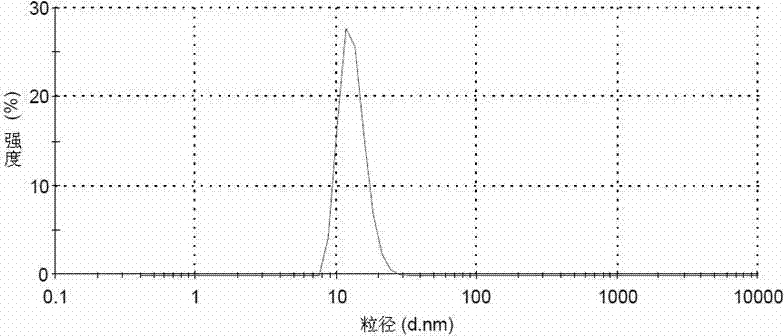
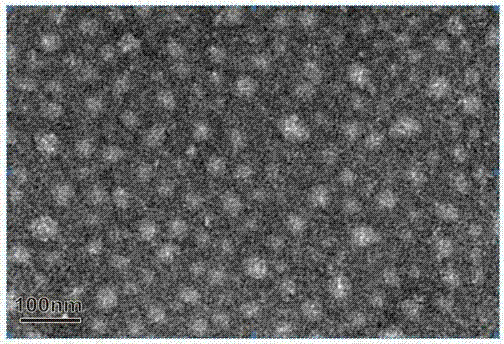
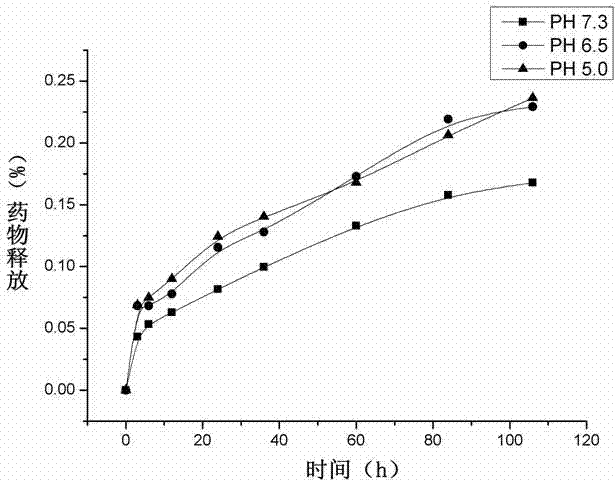
Image
Examples
Embodiment 1
[0036] Preparation of Carboxymethylcysteine Dimethyl Ester Hydrochloride
[0037]
[0038] Dissolve 10g of carboxymethylcysteine (SMC) in 100ml of anhydrous methanol (analytical pure), and ice-bath to below 0°C under the protection of nitrogen, slowly add thionyl chloride solution dropwise, keep the system temperature below 0°C and stir for a while Then rise to room temperature and react for 5 hours, remove methanol by rotary evaporation at 35°C to obtain mucus, wash twice with 100 ml of ether, and vacuum-dry at room temperature for 6 hours to obtain mucus carboxymethylcysteine dimethyl hydrochloride.
Embodiment 2
[0040] Preparation of polyglutamine carboxymethylcysteine dimethyl ester
[0041]
[0042]Sodium polyglutamate (molecular weight 35000, 4g, 26.4mmol), 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (15.23g, 79.45mmol), 1-hydroxy Benzotriazole (4.3g, 31.82mmol), carboxymethylcysteine dimethyl hydrochloride (13.77g, 56.67mmol) were dissolved in 200ml of anhydrous N,N-dimethylformamide (analytical pure) , under nitrogen protection, and stirred at 25°C for 48 hours. The solution after the reaction was slowly poured into stirred ice water to precipitate a white precipitate, which was filtered, washed three times with 200 ml of deionized water, and freeze-dried to obtain a white solid polyglutamine carboxymethyl cysteine dimethyl.
Embodiment 3
[0044] Preparation of polyglutamine carboxymethylcysteine
[0045]
[0046] Polyglutaminecarboxymethylcysteine dimethyl ester (1g, 3.14mmol per unit) was dissolved in 60ml of tetrahydrofuran (chemically pure), 60ml of 1mol / L lithium hydroxide aqueous solution was added, stirred for 8 hours overnight, and the tetrahydrofuran was removed by rotary evaporation. The remaining aqueous solution was adjusted to pH 3 with 2mol / L hydrochloric acid aqueous solution, poured into a dialysis bag (molecular weight cut-off 10000) and dialyzed for 24 hours, during which 4 liters of deionized water were replaced 4 times, and the resistivity of the aqueous solution outside the dialysis bag was detected. When the rate is lower than 0.1 mS / cm, collect the remaining solution in the dialysis bag, filter it with a 0.45 μm filter head, and freeze-dry to obtain white solid polyglutamine carboxymethylcysteine.
[0047] The obtained polyglutamine carboxymethylcysteine is dissolved with deuterated...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com