Agarose microsphere-containing cross-linked sodium hyaluronate gel for injection and preparation method thereof
A technology for cross-linking hyaluronic acid and agarose microspheres, which is applied in the fields of medical beauty and biomedicine, can solve the problems of increasing the burden on consumers and high cost of use by consumers, and achieves the effect of long-lasting shaping and prolonging the degradation cycle.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
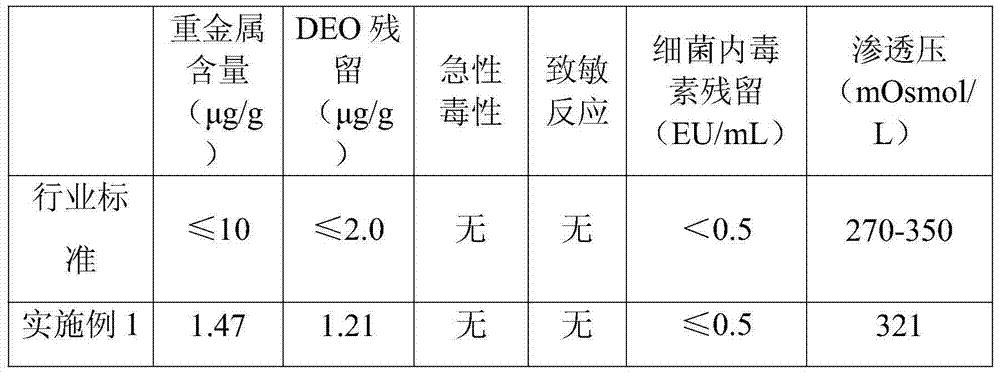
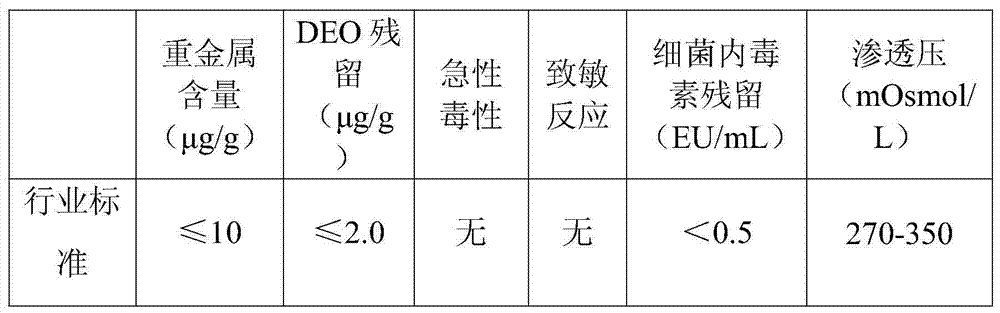
[0022] First add 0.1g of agarose microspheres and 10mL of water for injection into a clean beaker with a volume of 50mL. After simple stirring, add 2.5g of injection-grade sodium hyaluronate powder, and stir until the sodium hyaluronate is completely swollen. Add 15mL of sodium hydroxide solution prepared with water for injection and 1.2g of 1,2,7,8-dioxoctane (DEO) to the above-mentioned swollen sodium hyaluronate solution, and stir carefully until the final hydrogen The concentration of sodium oxide is 0.3mol / L. Then the evenly stirred mixture was placed in water at 50°C and heated for 4 hours to obtain a pale yellow gel with certain elasticity. Then cut the obtained gel into small pieces with a scalpel blade, wash with 0.05mol / L dilute hydrochloric acid at room temperature for 5 hours, and start washing with water for injection after washing. Once, the injection water was changed 10 times in total. Pass the washed gel twice through a sieve made of 35-mesh stainless steel ...
Embodiment 2
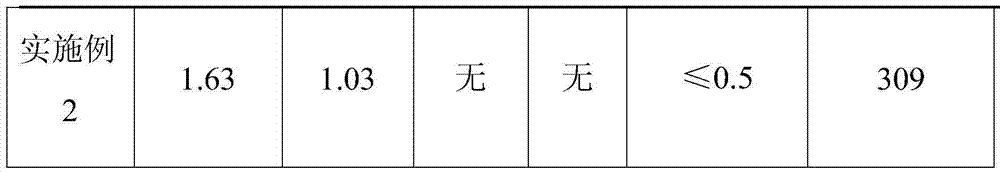
[0027] First add 0.5g of agarose microspheres and 15mL of water for injection into a clean beaker with a volume of 50mL, and then add 1.5g of injection-grade sodium hyaluronate powder after simple stirring, and stir until the sodium hyaluronate is completely swollen; Then add 10mL of sodium hydroxide solution prepared with water for injection and 0.6g of 1,2,7,8-diepoxyoctane (DEO) to the sodium hydroxide solution, and stir carefully until the final sodium hydroxide concentration is 0.1mol / L, place the evenly stirred mixture in water at 40°C and heat for 10 hours to obtain a pale yellow gel with certain elasticity. Cut the obtained gel into small pieces with a scalpel, wash with 0.05mol / L dilute hydrochloric acid at room temperature for 5 hours, and then change to water for injection to start washing at a washing temperature of 40°C, changing the water once every 1 hour, and changing the water 10 times in total ; Pass the washed gel through a 35-mesh stainless steel screen tw...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com