Separation detection method of pregabalin chiral isomers
A chiral isomer, pregabalin technology, applied in the field of analytical chemistry, can solve the problems of small sample volume, weak UV absorption, troublesome post-processing, etc., and achieve mild reaction conditions, high analytical sensitivity, and strong UV absorption. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] (1) Instruments and conditions
[0025] Mobile phase: n-hexane-absolute ethanol=85:15
[0026] Detection wavelength: 208nm
[0027] Column: AD-H 250×4.5mm, 5μm
[0028] Flow rate: 1.0ml / min
[0029] Column temperature: 35°C
[0030] Injection volume: 20ul
[0031] (2) Experimental steps
[0032] Take 25 mg of pregabalin mixture, add 50 mg of fluorenylmethoxycarbonyl chloride, put it in a 50 ml volumetric flask, add absolute ethanol for ultrasonic dissolution, then dilute to the mark with absolute ethanol and shake well, as a system suitability solution.
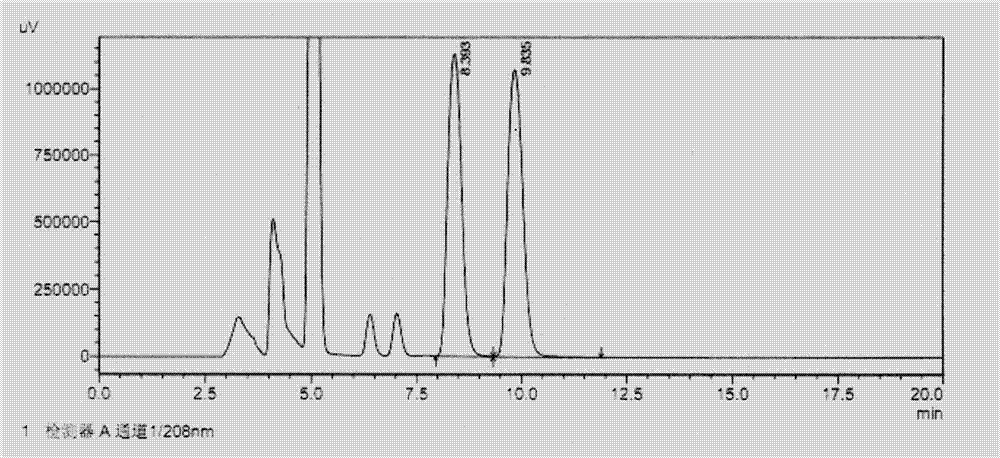
[0033] Take the system suitability solution and analyze it under the above conditions. see results figure 1 , where the retention time is 8.393min for pregabalin, where the retention time is 9.835min for pregabalin isomers, and the remaining peaks are peaks of unreacted derivative reagents, and the separation between the two is 2.352.
Embodiment 2
[0035] (1) Instruments and conditions
[0036] Mobile phase: n-hexane-absolute ethanol=95:5
[0037] Detection wavelength: 205nm
[0038] Column: AD-H 250×4.5mm, 5μm
[0039] Flow rate: 1.0ml / min
[0040] Column temperature: 25°C
[0041] Injection volume: 20ul
[0042] (2) Experimental steps
[0043] Take 25mg of pregabalin mixture, add 75mg of fluorenylmethoxycarbonyl chloride, put it in a 50ml volumetric flask, add 20ml of absolute ethanol for ultrasonic dissolution, then dilute to the mark with n-hexane and shake well as a system suitability solution.
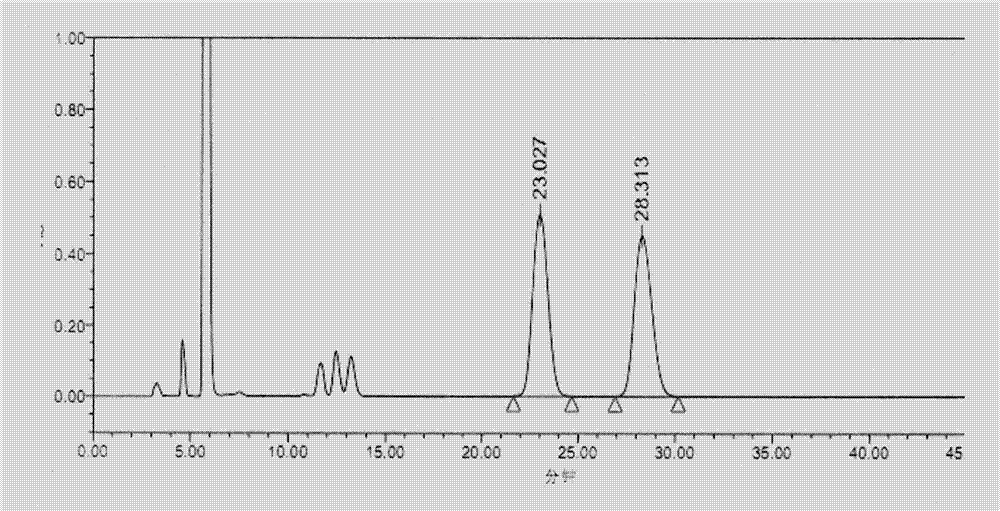
[0044] Take the system suitability solution and analyze it under the above conditions. see results figure 2 , where the retention time is 23.027min is pregabalin, where the retention time is 28.313min is pregabalin isomers, and the remaining peaks are peaks of unreacted derivative reagents, and the resolution is 3.32.
Embodiment 3
[0046] (1) Instruments and conditions
[0047] Mobile phase: n-hexane-dehydrated ethanol-dehydrated methanol=90:5:5
[0048] Detection wavelength: 210nm
[0049] Column: AD-H 250×4.5mm, 5μm
[0050] Flow rate: 0.8ml / min
[0051] Column temperature: 25°C
[0052] Injection volume: 20ul
[0053] (2) Experimental steps
[0054] Take 25mg of pregabalin mixture, add 100mg of fluorenylmethoxycarbonyl chloride, put it in a 50ml volumetric flask, add 20ml of absolute ethanol for ultrasonic dissolution, then dilute to the mark with n-hexane and shake well, as a system suitability solution.
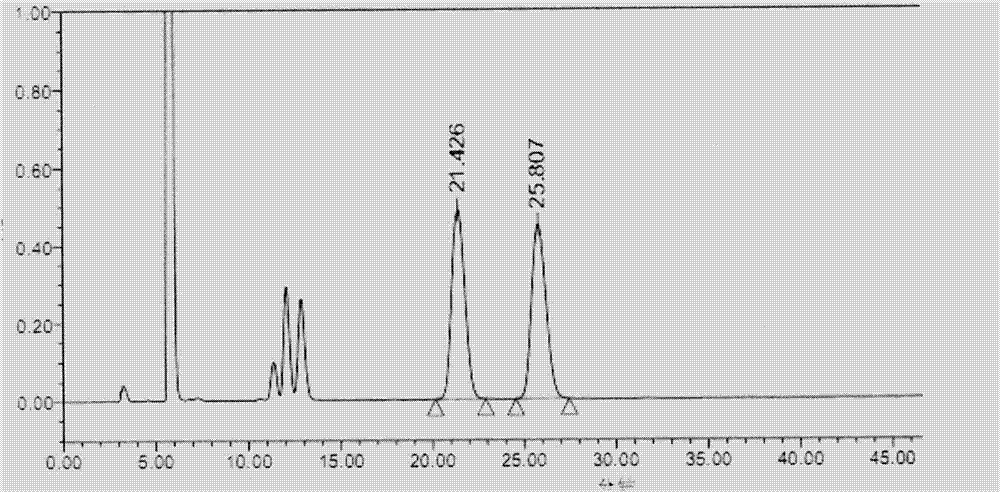
[0055] Take the system suitability solution and analyze it under the above conditions. see results image 3 , where the retention time is 21.426min is pregabalin, where the retention time is 25.807min is pregabalin isomers, and the remaining peaks are the peaks of unreacted derivative reagents, and the resolution is 3.09.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| separation | aaaaa | aaaaa |
| separation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com