Method for preparing C3N4 material with N defects and application
A C3N4, defect technology, applied in the field of preparation of nanomaterials, can solve the problems of limiting photocatalytic activity, energy consumption and time-consuming, and achieve the effect of major application prospects, good light absorption performance, and mild conditions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] A C with N deficiency 3 N 4 The preparation method of photocatalytic material comprises the following steps:
[0059] 1) Dissolve 15g of urea in 50mL of water, then add 10mL of KOH (0.001g / mL) aqueous solution, sonicate for 10 minutes to dissolve, then dry the above mixture in an oven at 80°C overnight;
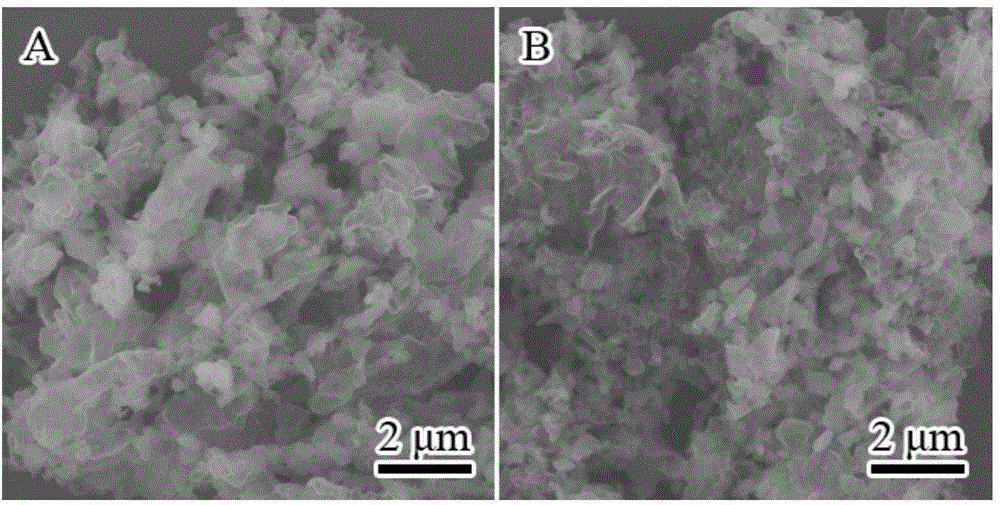
[0060] 2) The dried solid was put into a 100mL magnetic crucible, and calcined in a muffle furnace at 550°C for 4h, wherein the heating rate of the muffle furnace was 5°C min -1 ; get C with N deficiency 3 N 4 , named g-C 3 N x, Its scanning electron microscope pictures are as figure 1 Shown in B.
Embodiment 2
[0071] The comparative example in Example 1 was applied to the experiment of visible light photocatalytic water splitting to produce hydrogen.
[0072] 1) Determine C 3 N 4 As a catalyst, the amount of hydrogen produced and C 3 N 4 The relationship between dosage. Take 1,2,3,4,5,10,15,20,25,30mg Comparative Example C 3 N 4 Put into ten 60mL quartz tubes and add 20mL of lactic acid-water mixture with a volume ratio of 1:3. Nitrogen was passed into the quartz test tube for 30 minutes to remove the oxygen in the tube and sealed with a rubber stopper, and it was stirred and illuminated for 10 hours at room temperature. The light source is a 300W xenon lamp, and a 400nm filter is added to filter out ultraviolet light with a wavelength less than 400nm. The amount of hydrogen produced is measured with Shimadzu GC-2014 gas chromatograph every 1h, such as Image 6 shown. Before illumination, add 1wt% Pt precursor (H 2 PtCl 6 ), nitrogen was passed through for 30 minutes to e...
Embodiment 3
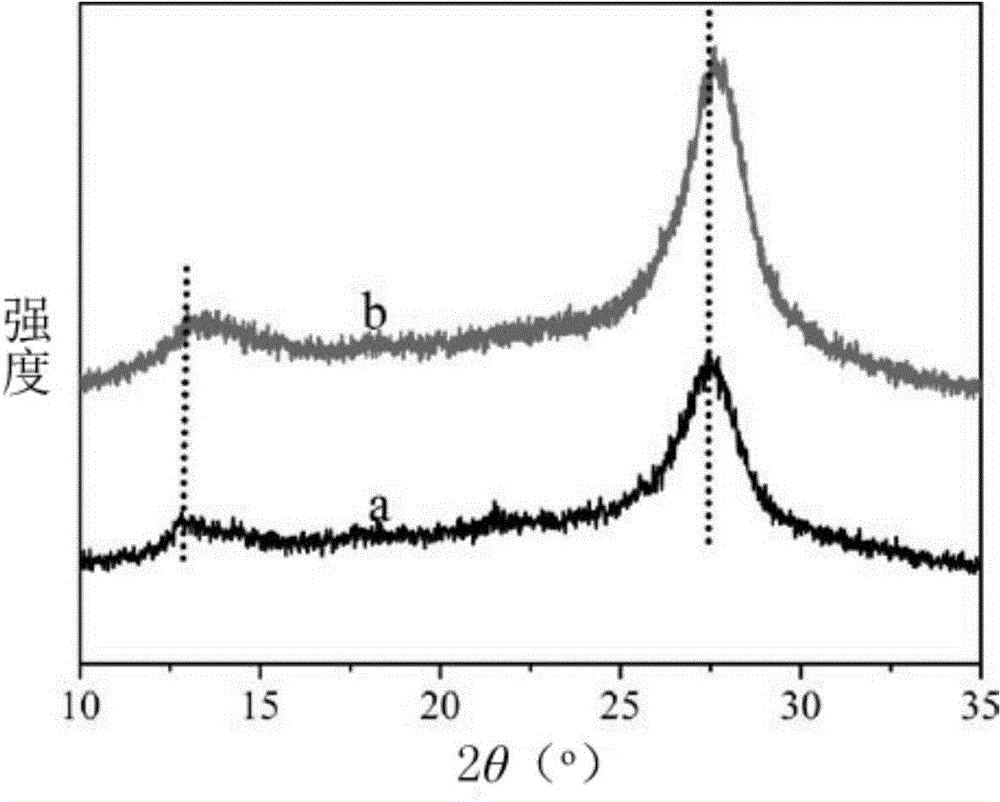
[0080] Repeat embodiment 1, prepare the catalyst of 0.01mg / mL (comparative example C 3 N 4 and C with N deficiency 3 N 4 ) aqueous solution, utilize the Hitachi-F4600 fluorescence spectrum to detect its fluorescence intensity under excitation at 380 nanometers, such as Figure 9 shown.
[0081] The results showed that compared to the comparative example C 3 N 4 ( Figure 9 a curve), C with N defects 3 N 4 ( Figure 9 The fluorescence intensity of curve b) is significantly reduced, which shows that the separation speed of photogenerated electrons and holes of the material has been improved to a certain extent.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com