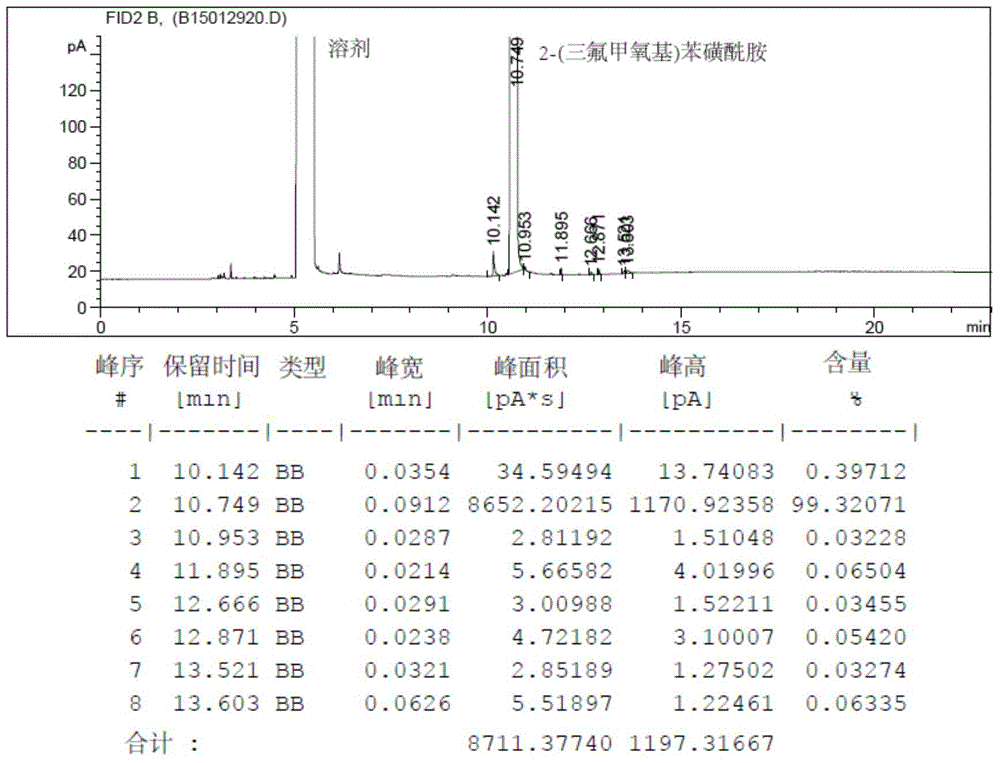
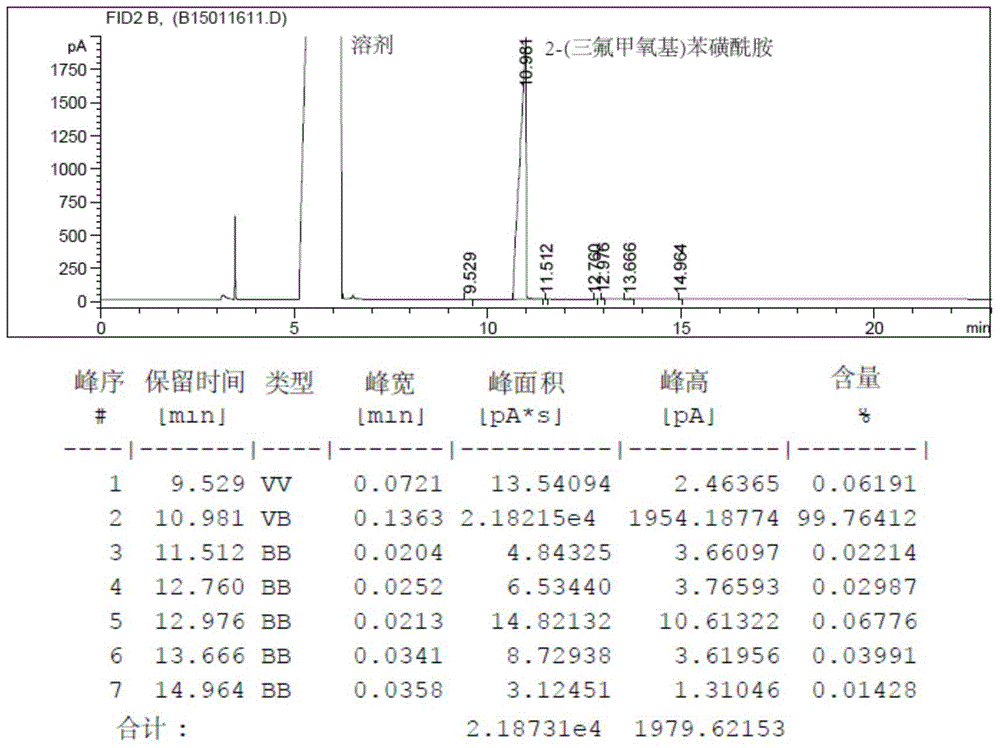
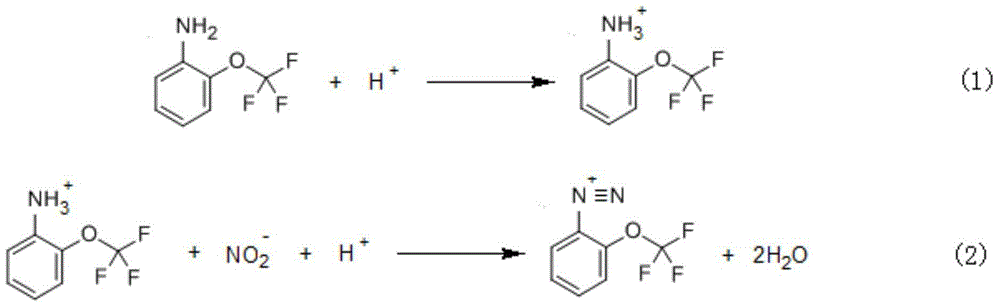
Method for preparing 2-(trifluoromethoxy) benzene sulfonamide
A technology of trifluoromethoxyl and benzenesulfonamide, which is applied in the field of preparation of 2-benzenesulfonamide, can solve the problems affecting the quality of the final product, low product yield, ammonia gas harming the environment and human health, and achieves a feasible process , high product purity, good environmental effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] 1. The preparation of 2-(trifluoromethoxy)aniline hydrochloride:
[0050] In a 1000mL four-necked flask, add 200g (1.92mol) of hydrochloric acid, 100g (0.565mol) of o-amino(trifluoromethoxy)benzene and 80g of water in sequence at room temperature, stir for 1 hour, and control the reaction temperature at 0-30°C. 380 g of a salt-forming reaction solution containing 2-(trifluoromethoxy)aniline hydrochloride was obtained.
[0051] ② Preparation of 2-(trifluoromethoxy)benzenediazonium salt:
[0052] Under stirring, add 120g (0.579mol) sodium nitrite aqueous solution dropwise to the 380g salt-forming reaction solution obtained in step ①, the reaction temperature is controlled at -20~5°C, the dropwise addition is completed in 1h, and the temperature is kept for 1h to obtain 500g of sodium nitrite containing 2 -Diazotization reaction solution of (trifluoromethoxy)benzene diazonium salt.
[0053] ③The preparation of 2-(trifluoromethoxy)benzenesulfonyl chloride:
[0054] In a ...
Embodiment 2
[0059] 1. The preparation of 2-(trifluoromethoxy) aniline sulfate:
[0060] In a 1000mL four-necked flask, add 62.2g (0.622mol) of concentrated sulfuric acid, 100g (0.565mol) of o-amino(trifluoromethoxy)benzene and 100g of water in turn at room temperature, stir for 1 hour, and control the reaction temperature at 80-100 °C, 262 g of a salt-forming reaction solution containing 2-(trifluoromethoxy)aniline sulfate was obtained.
[0061] ② Preparation of 2-(trifluoromethoxy)benzenediazonium salt:
[0062] Under stirring, add 385g (0.678mol) potassium nitrite aqueous solution dropwise to the 262g salt-forming reaction solution obtained in step ①, the reaction temperature is controlled at 40-50°C, the dropwise addition is completed in 3h, and the temperature is kept for 1h to obtain 646g The diazotization reaction solution of (trifluoromethoxy)benzene diazonium salt.
[0063] ③The preparation of 2-(trifluoromethoxy)benzenesulfonyl chloride:
[0064] In a 2000mL four-neck bottle, ...
Embodiment 3
[0068] 1. Preparation of 2-(trifluoromethoxy)aniline phosphate:
[0069] In a 1000mL four-neck flask, add 651g (5.65mol) of phosphoric acid and 100g (0.565mol) of o-amino(trifluoromethoxy)benzene at room temperature, and stir for 1 hour. The reaction temperature is controlled at 50-60°C to obtain 750g of Salt-forming reaction solution of 2-(trifluoromethoxy)aniline sulfate.
[0070] ② Preparation of 2-(trifluoromethoxy)benzenediazonium salt:
[0071] Under stirring, add 117g (0.566mol) sodium nitrite aqueous solution dropwise to the 750g salt-forming reaction solution obtained in step ①, the reaction temperature is controlled at 45-50°C, the dropwise addition is completed in 2h, and the temperature is kept for 1h to obtain 867g containing 2- The diazotization reaction solution of (trifluoromethoxy)benzene diazonium salt.
[0072] ③The preparation of 2-(trifluoromethoxy)benzenesulfonyl chloride:
[0073] In a 2000mL four-necked bottle, add 117g (1.13mol) of hydrochloric acid...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com