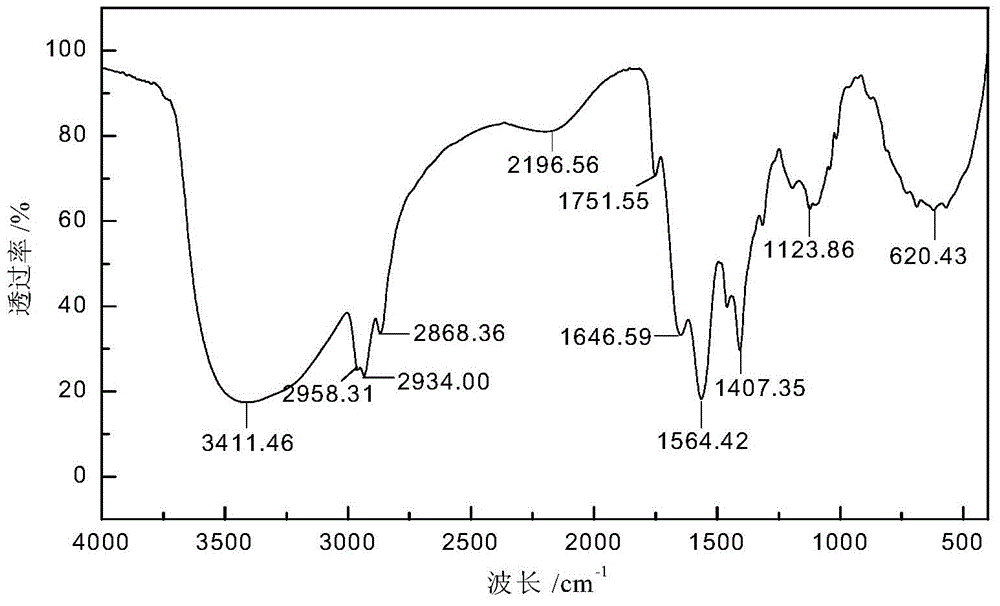
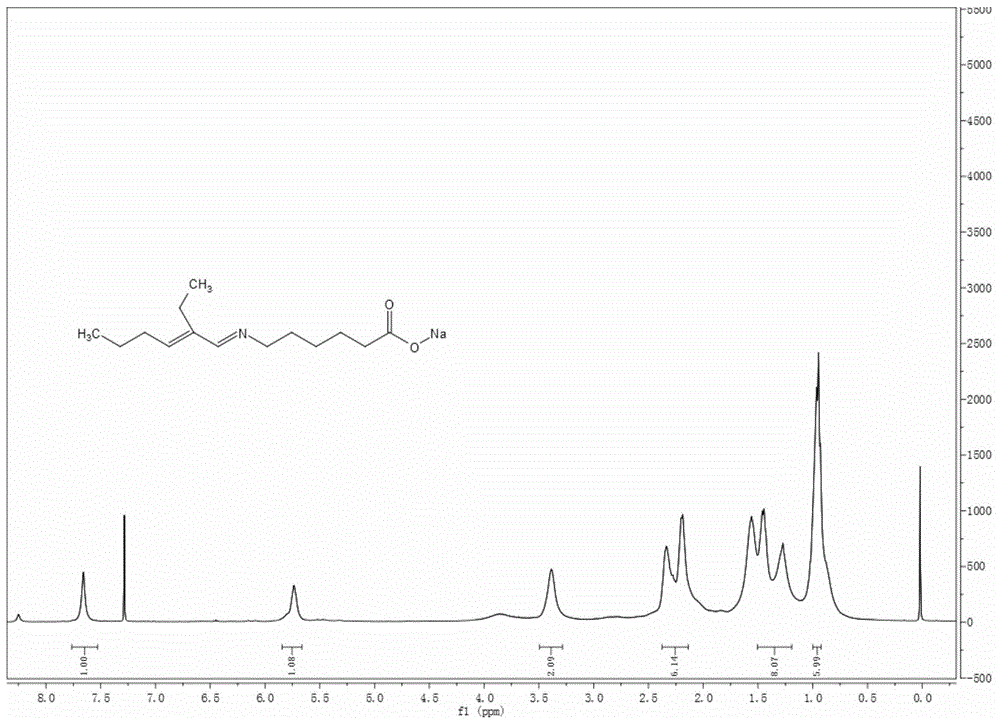
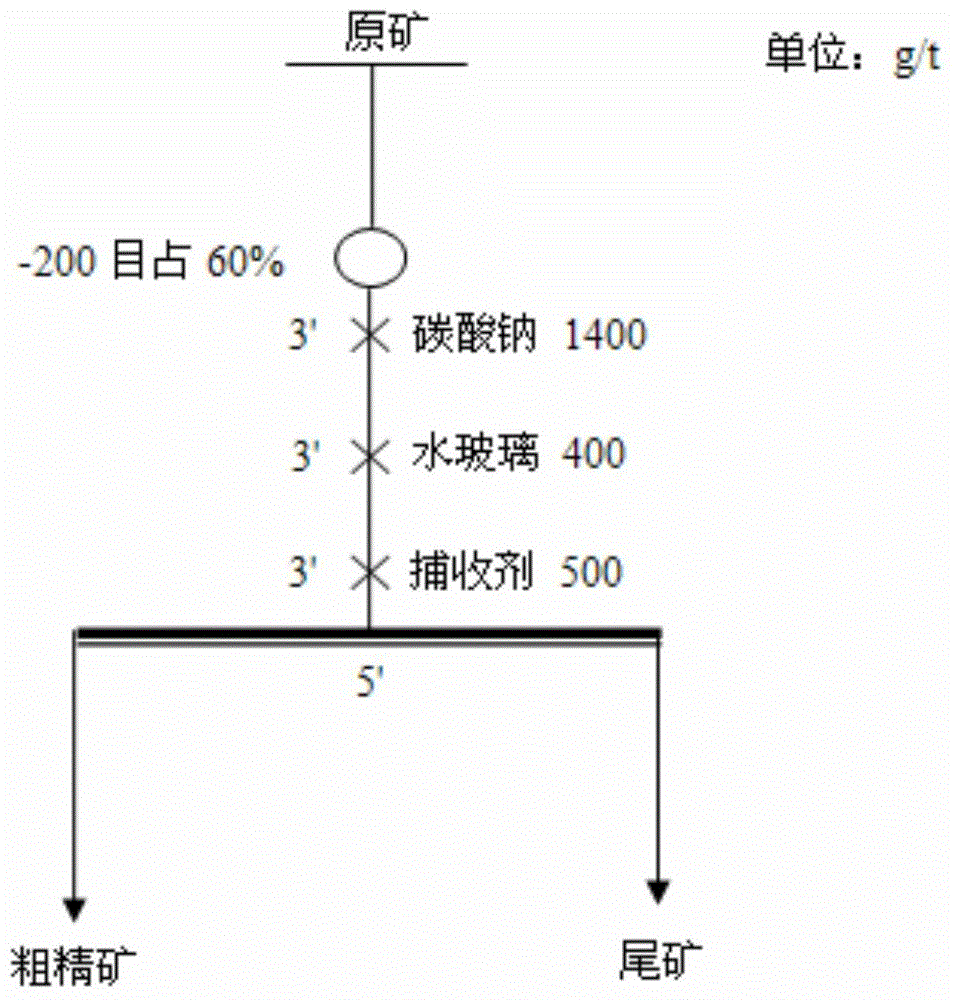
Schiff base caproate mineral flotation collecting agent as well as preparation method and application thereof
A mineral flotation and Schiff base technology, applied in flotation, solid separation and other directions, can solve the problems of poor frost resistance, poor dispersion and low selectivity, and achieve poor frost resistance and good selectivity. , the effect of strong collecting ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Preparation of sodium 6-(2-ethyl-2-enhexyl)iminocaproate:
[0056] Add 5.66 parts of caprolactam into the reactor with a stirring device, melt it at a temperature of 80°C, and dissolve 2 parts of solid sodium hydroxide in 3 parts of distilled water to make an alkali solution, and add the alkali solution Into the melted caprolactam liquid, raise the temperature to 100°C, stir for 2 hours, and the white solid precipitated after cooling to room temperature is sodium 6-aminocaproate.
[0057] In the reactor, add 7.56 parts of 2-ethyl-2-hexenal, 0.48 parts of p-toluenesulfonic acid, 60 parts of toluene and the 6-aminocaproic acid sodium obtained by the above-mentioned hydrolysis, and install the water separator. React for 3 hours at a reaction temperature of 115°C. After removing the toluene by distillation under reduced pressure, the solid was washed twice with anhydrous ether and acetone in turn, and the obtained orange-red solid was dried in a vacuum dryer at 50°C for 24 ...
Embodiment 2
[0059] Preparation of sodium 6-(2-ethyl-2-enhexyl)iminocaproate:
[0060] Add 11.32 parts of caprolactam into the reactor with a stirring device, melt it at a temperature of 80°C, and dissolve 4 parts of solid sodium hydroxide in 6 parts of distilled water to make an alkali solution, and add the alkali solution to Into the melted caprolactam liquid, raise the temperature to 100°C, stir for 2 hours, and the white solid precipitated after cooling to room temperature is sodium 6-aminocaproate.
[0061] In the reactor, add 15.12 parts of 2-ethyl-2-hexenal, 0.95 parts of p-toluenesulfonic acid, 80 parts of toluene and the 6-aminocaproic acid sodium obtained by the above-mentioned hydrolysis successively, install the water separator, React for 4 hours at a reaction temperature of 115°C. After toluene was removed by distillation under reduced pressure, the solid was washed twice with anhydrous ether and acetone in turn, and the obtained orange-red solid was dried in a vacuum dryer at...
Embodiment 3
[0063] Preparation of sodium n-octyliminocaproate:
[0064] Add 11.32 parts of caprolactam into the reactor with a stirring device, melt it at a temperature of 80°C, and dissolve 4 parts of solid sodium hydroxide in 6 parts of distilled water to make an alkali solution, and add the alkali solution to Into the melted caprolactam liquid, raise the temperature to 100°C, stir for 2 hours, and the white solid precipitated after cooling to room temperature is sodium 6-aminocaproate.
[0065] Add 15.12 parts of n-octanal, 0.95 parts of p-toluenesulfonic acid, and 80 parts of toluene to the reactor in sequence, and drop the molten sodium 6-aminocaproate liquid prepared by the above hydrolysis at 100°C. After the addition is completed, Install a water separator, react at a reaction temperature of 115°C for 4 hours, and distill off the toluene under reduced pressure, wash the solid twice with anhydrous ether and acetone in turn, and dry the obtained yellow solid in a vacuum dryer at 50°...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com